��Ŀ����
����Ŀ������ˮƽ�Ļ����ڳߴ硢���ܵȷ�������������������Ա�����ص㣬��ѧ���ǶԷ���������о�ȡ���˽ϴ��չ������ϩ��һ�ֵ���ת�����������еĹؼ��������AΪԭ�Ϻϳ�����ϩ�ĺϳ�·��֮һ�����ͼ��

��֪����R-NO2![]() R-NH2
R-NH2
��![]() +
+![]()
![]()
![]() (Diels-Alder��Ӧ)
(Diels-Alder��Ӧ)
�ش��������⣺
(1)A��������ͼ������ʺɱ�Ϊ_____��E�Ľṹ��ʽΪ____________��
(2)��Ӧ��Ļ�ѧ��Ӧ������_______����Ӧ���뷴Ӧ���ܷ�Ե�?��˵�����ɣ�_________��
(3)F��G�����ʵ���֮��1:1������Ӧ�����÷�Ӧ�Ļ�ѧ����ʽ��____________��
(4)ʵ���ҳ���˳��ϩ������![]() ��KOH��Һ��ȥ����ϩ�����еĻ�����G��������G������������ˮ������______(��ṹ��ʽ)����ȥ��
��KOH��Һ��ȥ����ϩ�����еĻ�����G��������G������������ˮ������______(��ṹ��ʽ)����ȥ��
(5)X��D������ͬ�����ţ�����Է���������D���14����X��ͬ���칹���к�����̼ԭ�ӵ���________(��ṹ��ʽ)��
(6)д���Լױ����Ҵ�Ϊԭ���Ʊ��ֲ�����ҩ��������(![]() )�ĺϳ�·��______ (���Լ���ѡ)��
)�ĺϳ�·��______ (���Լ���ѡ)��![]()
![]() ����
����![]()
���𰸡�78  ȡ����Ӧ ���ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ���������
ȡ����Ӧ ���ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ��������� 



��������
�ɿ�ͼ �е�D�Ľṹ��ʽ���ƿ�֪��C�Ľṹ��ʽΪ
�е�D�Ľṹ��ʽ���ƿ�֪��C�Ľṹ��ʽΪ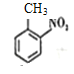 ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ![]() ��A�Ľṹ��ʽΪ
��A�Ľṹ��ʽΪ![]() ��������֪��R-NO2
��������֪��R-NO2![]() R-NH2������E�Ľṹ��ʽΪ
R-NH2������E�Ľṹ��ʽΪ �����ݿ�ͼF�Ľṹ��ʽΪ
�����ݿ�ͼF�Ľṹ��ʽΪ ������ϩ�Ľṹ��ʽΪ
������ϩ�Ľṹ��ʽΪ ��F��G�����ʵ���֮��1:1������Ӧ������
��F��G�����ʵ���֮��1:1������Ӧ������ ������G�Ľṹ��ʽΪ
������G�Ľṹ��ʽΪ ���Դ˷������
���Դ˷������
(1)��������A�Ľṹ��ʽΪ![]() ������A��������ͼ������ʺɱ�Ϊ78����D�Ľṹ��ʽΪ
������A��������ͼ������ʺɱ�Ϊ78����D�Ľṹ��ʽΪ ��������֪��R-NO2
��������֪��R-NO2![]() R-NH2������E�Ľṹ��ʽΪ
R-NH2������E�Ľṹ��ʽΪ ���ʴ𰸣�78��
���ʴ𰸣�78�� ��
��
(2)��������A�Ľṹ��ʽΪ![]() ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ![]() �����Է�Ӧ������ȡ����Ӧ����Ӧ���Ǽױ���������Ӧ����Ӧ���ǰ�����ת��Ϊ�������Ƕ����ı��������Բ��ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ��������ᣬ�ʴ𰸣�ȡ����Ӧ�����ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ��������
�����Է�Ӧ������ȡ����Ӧ����Ӧ���Ǽױ���������Ӧ����Ӧ���ǰ�����ת��Ϊ�������Ƕ����ı��������Բ��ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ��������ᣬ�ʴ𰸣�ȡ����Ӧ�����ܶԵ�������ߵ���Ӧ����˳������KMnO4��Һ��������ͬʱ����Ҳ�����������õ��ڰ��������
(3)���ݿ�ͼF�Ľṹ��ʽΪ ��F��G�����ʵ���֮��1:1������Ӧ������
��F��G�����ʵ���֮��1:1������Ӧ������ ������G�Ľṹ��ʽΪ
������G�Ľṹ��ʽΪ ���÷�Ӧ�Ļ�ѧ����ʽ��
���÷�Ӧ�Ļ�ѧ����ʽ�� ���ʴ𰸣�
���ʴ𰸣� ��
��
(4) ����ϩ�Ľṹ��ʽ�� ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ ��ʵ���ҳ���˳��ϩ������
��ʵ���ҳ���˳��ϩ������![]() ��KOH��Һ��ȥ�����еĻ�����G��������G������������ˮ������
��KOH��Һ��ȥ�����еĻ�����G��������G������������ˮ������ ���ʴ𰸣�
���ʴ𰸣� ��
��
(5) ��D�Ľṹ��ʽΪ ��X��D������ͬ�����ţ�����Է���������D���14������X��D��һ��-CH2����X��ͬ���칹���к�����̼ԭ�ӵ���
��X��D������ͬ�����ţ�����Է���������D���14������X��D��һ��-CH2����X��ͬ���칹���к�����̼ԭ�ӵ��� ���ʴ𰸣�
���ʴ𰸣�  ��
��
(6)�Լױ����Ҵ�Ϊԭ���Ʊ��ֲ�����ҩ��������(![]() )�ĺϳ�·�ߣ�
)�ĺϳ�·�ߣ� ��
��