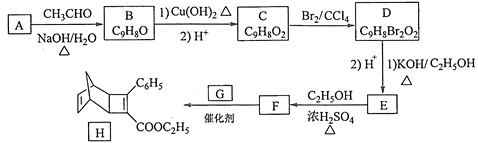
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ‘ΤΡœ «ΡœΖΫΥΩ≥ώ÷°¬ΖΒΡ÷Ί“ΣΫΎΒψ,”–Ή≈ΖαΗΜΒΡΆ≠ΡχΩσ ·Ή ‘¥ΓΘΜΊ¥πœ¬Ν–œύΙΊΈ ΧβΘΚ
(1)Ρχ‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «____,ΤδΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ_____,ΗΟ‘≠Ή”ΚΥΆβ”–___ΗωΈ¥≥…Ε‘ΒγΉ”ΓΘ
(2)Ni(NH3)4SO4÷–NΒΡ‘”Μ·ΙλΒάάύ–Ά «_____;1 mol Ni(NH3)42+÷–Κ§”–ΒΡ![]() Φϋ ΐΡΩΈΣ____Ηω;SO42-ΒΡΝΔΧεΙΙ–Ά «______ΓΘ
Φϋ ΐΡΩΈΣ____Ηω;SO42-ΒΡΝΔΧεΙΙ–Ά «______ΓΘ
(3)Α± «_____Ζ÷Ή”(ΧνΓΑΦΪ–‘Γ±ΜρΓΑΖ«ΦΪ–‘Γ±),Ψ≠≤βΕ®NH4FΈΣΖ÷Ή”ΨßΧε,άύ±»NH3ΓΛH2O±μ Ψ≥ωNH4FΖ÷Ή”÷–ΒΡ«βΦϋ_____ΓΘ
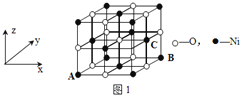
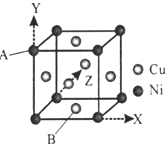
(4)Ά≠ΡχΚœΫπΒΡΝΔΖΫΨßΑϊΫαΙΙ»γΆΦΥυ Ψ,Τδ÷–‘≠Ή”AΒΡΉχ±ξ≤Έ ΐΈΣ(0,1,0);
ΔΌ‘≠Ή”BΒΡΉχ±ξ≤Έ ΐΈΣ____;
ΔΎ»τΗΟΨßΧεΟήΕ»ΈΣdg/cm3,‘ρΆ≠Ρχ‘≠Ή”ΦδΉνΕΧΨύάκΈΣ____pm
ΓΨ¥πΑΗΓΩΒΎΥΡ÷ήΤΎΔχΉε 1s22s22p63s23p63d84s2 2 sp3 16NA(Μρ9.632ΓΝ1024) ’ΐΥΡΟφΧε–Έ ΦΪ–‘ F-HΓΛΓΛΓΛNΘ®ΜρN-HΓΛΓΛΓΛFΘ© ![]()
![]()
ΓΨΫβΈωΓΩ
Θ®1Θ©Ρχ‘≠Ή”–ρ ΐΈΣ28Θ§Ψί¥Υ≈–ΕœΤδ‘Ύ÷ήΤΎ±μ÷–ΈΜ÷ΟΘΜ”…ΙΙ‘λ‘≠άμ ι–¥‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΘΜ≈–Εœ‘≠Ή”ΚΥΆβΈ¥≥…Ε‘ΒγΉ”ΘΜ
Θ®2Θ©“άΨίΦέ≤ψΒγΉ”ΜΞ≥βάμ¬έΦΤΥψ≈–ΕœΦ¥Ω…ΘΜ
1 mol Ni(NH3)42+÷–Κ§”–ΒΡ![]() Φϋ ΐΡΩΈΣΘ®3ΓΝ4+4Θ©NA=16NAΘΜ
Φϋ ΐΡΩΈΣΘ®3ΓΝ4+4Θ©NA=16NAΘΜ
“άΨίΦέ≤ψΒγΉ”ΜΞ≥βάμ¬έΦΤΥψ≈–ΕœΦ¥Ω…ΘΜ
Θ®3Θ©Α±Ζ÷Ή”÷–”–“ΜΕ‘Ρ©≥…ΦϋΒγΉ”Ε‘Θ§ «ΦΪ–‘Ζ÷Ή”ΘΜNH4FΖ÷Ή”÷–ΒΡ«βΦϋF-HΓΛΓΛΓΛNΘ®ΜρN-HΓΛΓΛΓΛFΘ©ΘΜ
Θ®4Θ©ΔΌ‘≠Ή”BΒΡΉχ±ξ≤Έ ΐΈΣ![]() ΘΜ
ΘΜ
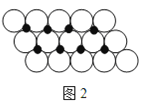
ΔΎ¥Π”ΎΟφΕ‘Ϋ«œΏ…œΒΡNiΓΔCu‘≠Ή”÷°ΦδΨύάκΉνΫϋΘ§ΨßΑϊΟφΕ‘Ϋ«œΏ≥ΛΕ»Β»”ΎNiΓΔCu‘≠Ή”ΨύάκΒΡ2±ΕΘ§ΕχΟφΕ‘Ϋ«œΏ≥ΛΕ»Β»”ΎΨßΑϊάβ≥ΛΒΡ![]() ±ΕΘ§ΨυΧ·Ζ®ΦΤΥψΨßΑϊ÷–NiΓΔCu‘≠Ή” ΐΡΩΘ§ΦΤΥψΨßΑϊΒΡ÷ ΝΩΘ§ΕχΨßΑϊ÷ ΝΩ=ΨßΧεΟήΕ»ΓΝΨßΑϊΧεΜΐΓΘ
±ΕΘ§ΨυΧ·Ζ®ΦΤΥψΨßΑϊ÷–NiΓΔCu‘≠Ή” ΐΡΩΘ§ΦΤΥψΨßΑϊΒΡ÷ ΝΩΘ§ΕχΨßΑϊ÷ ΝΩ=ΨßΧεΟήΕ»ΓΝΨßΑϊΧεΜΐΓΘ
(1)Ρχ‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο «ΒΎΥΡ÷ήΤΎΔχΉεΘ§ΤδΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p63s23p63d84s2Θ§ΗΟ‘≠Ή”ΚΥΆβ”–2ΗωΈ¥≥…Ε‘ΒγΉ”ΓΘ
Θ®2Θ©Ni(NH3)4SO4÷–÷––Ρ‘≠Ή”NΒΡΦέΒγΉ”Ε‘ ΐΈΣΘ®5+3Θ©/2=4Θ§÷––Ρ‘≠Ή”ΈόΙ¬ΒγΉ”Ε‘Θ§Ι≤–Έ≥…4ΗωΠ“ΦϋΘ§ΗυΨίΦέ≤ψΒγΉ”Ε‘ΜΞ≥βάμ¬έΘ§Ω’ΦδΙΙ–ΆΈΣΥΡΟφΧε–ΆΘ§≤…”Οsp3‘”Μ·ΘΜ
1 mol Ni(NH3)42+÷–Κ§”–ΒΡ![]() Φϋ ΐΡΩΈΣΘ®3ΓΝ4+4Θ©NA=16NA(Μρ9.632ΓΝ1024)ΘΜ
Φϋ ΐΡΩΈΣΘ®3ΓΝ4+4Θ©NA=16NA(Μρ9.632ΓΝ1024)ΘΜ
SO42-ΒΡ÷––Ρ‘≠Ή”SΒΡΦέΒγΉ”Ε‘ ΐΈΣΘ®6+2Θ©/2=4Θ§÷––Ρ‘≠Ή”ΈόΙ¬ΒγΉ”Ε‘Θ§Ι≤–Έ≥…4ΗωΠ“ΦϋΘ§ΗυΨίΦέ≤ψΒγΉ”Ε‘ΜΞ≥βάμ¬έΘ§≤…”Οsp3‘”Μ·Θ§Ω’ΦδΙΙ–ΆΈΣ’ΐΥΡΟφΧε–ΆΘΜ
Θ®3Θ©Α±Ζ÷Ή”÷–”–“ΜΕ‘Ρ©≥…ΦϋΒγΉ”Ε‘Θ§ΤδΩ’ΦδΙΙ–ΆΈΣ»ΐΫ«ΉΕ–ΈΘ§ «ΦΪ–‘Ζ÷Ή”ΘΜNH4FΖ÷Ή”÷–ΒΡ«βΦϋF-HΓΛΓΛΓΛNΘ®ΜρN-HΓΛΓΛΓΛFΘ©ΘΜ
Θ®4Θ©ΔΌΗυΨίAΒψΒΡΉχ±ξΘ§Ω…“‘≈–ΕœΨßΑϊΒΉΟφΒΡΟφ–Ρ…œΒΡ‘≠Ή”BΒΡΉχ±ξ≤Έ ΐΈΣ![]() ΘΜ
ΘΜ
ΔΎ¥Π”ΎΟφΕ‘Ϋ«œΏ…œΒΡNiΓΔCu‘≠Ή”÷°ΦδΨύάκΉνΫϋΘ§…ηΕΰ’Ώ÷°ΦδΨύάκΈΣa cmΘ§ΨßΑϊΟφΕ‘Ϋ«œΏ≥ΛΕ»Β»”ΎNiΓΔCu‘≠Ή”ΨύάκΒΡ2±ΕΘ§ΕχΟφΕ‘Ϋ«œΏ≥ΛΕ»Β»”ΎΨßΑϊάβ≥ΛΒΡ![]() ±ΕΘ§Ι ΨßΑϊάβ≥Λ=2a cmΓΝ
±ΕΘ§Ι ΨßΑϊάβ≥Λ=2a cmΓΝ![]() /2=
/2=![]() a cmΘ§ΨßΑϊ÷ ΝΩ=(59+64ΓΝ3)/NAgΘ§Ι Θ®
a cmΘ§ΨßΑϊ÷ ΝΩ=(59+64ΓΝ3)/NAgΘ§Ι Θ®![]() a cmΘ©3ΓΝdgΓΛcmΘ≠3 =(59+64ΓΝ3)/NAgΘ§ΫβΒΟa=
a cmΘ©3ΓΝdgΓΛcmΘ≠3 =(59+64ΓΝ3)/NAgΘ§ΫβΒΟa=![]()

 Νι–«ΦΤΥψ–Γ¥ο»ΥœΒΝ–¥πΑΗ
Νι–«ΦΤΥψ–Γ¥ο»ΥœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΫαΚœΥυ―ßΡΎ»ίΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔώΘ°Ρ≥Ά§―ßΫχ––”Αœλ≤ίΥα”κΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΖ¥”ΠΥΌ¬ “ρΥΊΒΡ―–ΨΩΓΘ≤ίΥα”κΥα–‘ΗΏΟΧΥαΦΊΒΡΖ¥”ΠΈΣΘΚ2KMnO4+5H2C2O4+3H2SO4ΘΫK2SO4+2MnSO4+10CO2Γϋ+8H2OΓΘ “Έ¬œ¬Θ§ΝΫ÷ß ‘ΙήΖ÷±π±ύΚ≈ΔΌΚΆΔΎΘ§ Β―ι ΐΨί»γœ¬ΘΚ
Β―ι–ρΚ≈ | ΔΌ | ΔΎ |
Φ”»κ ‘ΦΝ | 4 mL 0.01mol/LΥα–‘ KMnO4»ή“Κ 2 mL 0.1mol/L H2C2O4»ή“Κ | 4 mL 0.01mol/LΥα–‘ KMnO4»ή“Κ 2 mL 0.1mol/L H2C2O4»ή“Κ “ΜΝΘΜΤΕΙΝΘ¥σΒΡMnSO4ΙΧΧε |
Ά …Ϊ ±Φδ/s | 116 | 6 |
‘ΙήΔΌ÷–KMnO4»ή“ΚΆ …ΪΒΡΥΌ¬ ΩΣ Φ °Ζ÷ΜΚ¬ΐΘ§“ΜΕΈ ±ΦδΚσΆΜ»ΜΦ”ΩλΓΘ
«κΜΊ¥πΘΚ
(1)ΗΟ Β―ιΫα¬έ «___________________________________________________________ΓΘ
(2) Β―ιΔΎ―Γ”ΟMnSO4ΙΧΧεΕχ≤Μ «MnCl2ΙΧΧεΒΡ‘≠“ρ «____________________________ΓΘ
(3)ΗΟΆ§―ßΈΣ Ι Β―ιΗϋΦ”―œΟήΘ§‘Ύ ‘ΙήΔέ÷–ΉωΝΥ»γœ¬ Β―ιΘ§«κ‘Λ≤βΆ …Ϊ ±Φδ‘ΦΈΣ_____ΓΘ
Β―ι–ρΚ≈ | Δέ |
Φ”»κ ‘ΦΝ | 4 mL 0.01mol/LΥα–‘ KMnO4»ή“Κ 2 mL 0.1mol/L H2C2O4»ή“Κ “ΜΝΘΜΤΕΙΝΘ¥σΒΡNa2SO4ΙΧΧε |
Ά …Ϊ ±Φδ/s |
ΔρΘ°ΒΈΕ®Ζ® «“Μ÷÷÷Ί“ΣΒΡΕ®ΝΩΖ÷ΈωΖΫΖ®Θ§”Π”ΟΖΕΈßΚήΙψΓΘΡ≥ΒΊ –≥Γ…œœζ έΒΡ“Μ÷÷ ≥”ΟΨΪ÷Τ―ΈΑϋΉΑ¥ϋ…œ”–»γœ¬≤ΩΖ÷ΥΒΟςΘΚ
≤ζΤΖΒ»ΦΕ | “ΜΦΕ |
≈δΝœ | ≥―ΈΓΔΒβΥαΦΊ(KIO3)ΓΔΩΙΫαΦΝ |
ΒβΚ§ΝΩ(“‘IΦΤ) | 20ΓΪ50 mgΓΛkgΘ≠1 |
“―÷ΣΘΚIO3Θ≠ΘΪ5IΘ≠ΘΪ6HΘΪ = 3I2ΘΪ3H2OΘ§I2ΘΪ2S2O32- = 2IΘ≠ΘΪS4O62-Ρ≥―ß…ζΡβ≤βΕ® ≥”ΟΨΪ÷Τ―ΈΒΡΒβΚ§ΝΩΘ§Τδ≤Ϋ÷ηΈΣ
aΘ°ΉΦ»Ζ≥Τ»ΓW g ≥―ΈΘ§Φ” ΝΩ’τΝσΥ° ΙΤδΆξ»Ϊ»ήΫβ
bΘ°”ΟœΓΝρΥαΥαΜ·ΥυΒΟ»ή“ΚΘ§Φ”»κΉψΝΩKI»ή“ΚΘ§ ΙKIO3”κKIΖ¥”ΠΆξ»Ϊ
cΘ°Φ”»κ÷Η ΨΦΝΘ§÷πΒΈΦ”»κΈο÷ ΒΡΝΩ≈®Ε»ΈΣ2.0ΓΝ10/span>Θ≠3 molΓΛLΘ≠1ΒΡNa2S2O3»ή“Κ10.0 mLΘ§«ΓΚΟΖ¥”ΠΆξ»Ϊ
(4)c÷–Φ”»κΒΡ÷Η ΨΦΝΩ…―Γ”Ο_________Θ§«ΓΚΟΆξ»ΪΖ¥”Π ±ΒΡœ÷œσ «_______________ΓΘ
(5)»τ≤ΌΉςb‘ΎΩ’Τχ÷–’ώΒ¥ ±ΦδΙΐ≥ΛΘ§‘ρΉν÷’≤βΕ®ΒΡ≤βΕ® ≥”ΟΨΪ÷Τ―Έ÷–ΒΡΒΡΒβΚ§ΝΩΜα__________(ΧνΓΑΤΪΗΏΓ±ΓΔΓΑΤΪΒΆΓ±ΜρΓΑΟΜ”–”ΑœλΓ±)ΓΘ
(6)ΗυΨί“‘…œ Β―ιΚΆΑϋΉΑΥΒΟςΘ§ΥψΒΟΥυ≤β ≥”ΟΨΪ÷Τ―ΈΒΡΒβΚ§ΝΩ «(“‘Κ§WΒΡ¥ζ ΐ Ϋ±μ Ψ)________mgΓΛkgΘ≠1(ΦΤΥψΫαΙϊ±ΘΝτ’ϊ ΐΦ¥Ω…)ΓΘ