��Ŀ����
(16��)̼������������������Ҫ�ķǽ���Ԫ�ء�
��1��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)�ġ�H����ֱ�Ӳ�����ԭ���� ��
��֪��a��2CO(g)+O2(g)=2CO2(g) ��H =-566��0 kJ��mol-1
b��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H =-890��0 kJ��mol-1
��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)���Ȼ�ѧ����ʽΪ ��
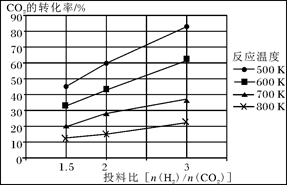
��2����ҵ�Ϻϳɰ����ķ�ӦΪ��N2(g)+3H2(g) 2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(
2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����( )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺

�ٷ�Ӧ�ﵽƽ��״̬Bʱ���������ݻ�10 L����T1ʱ���ϳɰ���Ӧ��ƽ�ⳣ��K= L2��mol-1��
��ƽ��״̬��A�䵽Cʱ����Ӧ��ƽ�ⳣ��K(A) K(C)(�>������<����=��)��
��3����25��ʱ��HSCN��HClO��H2CO3�ĵ��볣�����±���
��1 mol��L-1��KSCN��Һ�У��������ӵ�Ũ���ɴ�С��˳��Ϊ > > > ��
����Na2CO3��Һ�м������HClO��Һ����Ӧ�Ļ�ѧ����ʽΪ ��
��25��ʱ��Ϊ֤��HClOΪ���ᣬijѧϰС���ͬѧû������������ʵ�鷽�����������ַ����У�����Ϊ�ܹ��ﵽʵ��Ŀ�ĵ��� (�����и��������)��
a����pH�Ʋ���0��1 mol��L-1NaClO��Һ��pH�������pH>7����֤��HClOΪ����
b����pH��ֽ����0��01 mol��L-1HClO��Һ��pH�������pH>2����֤��HClOΪ����
c������������Ũ�Ⱦ�Ϊ0��1 mol��L-1��HClO��Һ������ĵ����ԣ������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ����
��1��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)�ġ�H����ֱ�Ӳ�����ԭ���� ��
��֪��a��2CO(g)+O2(g)=2CO2(g) ��H =-566��0 kJ��mol-1
b��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H =-890��0 kJ��mol-1
��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)���Ȼ�ѧ����ʽΪ ��
��2����ҵ�Ϻϳɰ����ķ�ӦΪ��N2(g)+3H2(g)
 2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(
2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����( )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
�ٷ�Ӧ�ﵽƽ��״̬Bʱ���������ݻ�10 L����T1ʱ���ϳɰ���Ӧ��ƽ�ⳣ��K= L2��mol-1��
��ƽ��״̬��A�䵽Cʱ����Ӧ��ƽ�ⳣ��K(A) K(C)(�>������<����=��)��
��3����25��ʱ��HSCN��HClO��H2CO3�ĵ��볣�����±���
| HClO | HSCN | H2CO3 |
| K=3.210-8 | K=0.13 | Kl=4.210-7 K2=5.610-11 |
��1 mol��L-1��KSCN��Һ�У��������ӵ�Ũ���ɴ�С��˳��Ϊ > > > ��
����Na2CO3��Һ�м������HClO��Һ����Ӧ�Ļ�ѧ����ʽΪ ��
��25��ʱ��Ϊ֤��HClOΪ���ᣬijѧϰС���ͬѧû������������ʵ�鷽�����������ַ����У�����Ϊ�ܹ��ﵽʵ��Ŀ�ĵ��� (�����и��������)��
a����pH�Ʋ���0��1 mol��L-1NaClO��Һ��pH�������pH>7����֤��HClOΪ����
b����pH��ֽ����0��01 mol��L-1HClO��Һ��pH�������pH>2����֤��HClOΪ����
c������������Ũ�Ⱦ�Ϊ0��1 mol��L-1��HClO��Һ������ĵ����ԣ������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ����
��1����5�֣����Կ��Ʒ�Ӧֻ����CO(g)��2�֣����ַ�Ӧ���Կ��Ƽ��ɣ�
2CH4(g)+3O2(g)=2CO(g)+4H2O(g) ?H=��1214.0kJ?mol?1��3�֣�
��2����4�֣���0.025��2�֣��� > ��2�֣�
��3����7�֣��� c(K+)>C(SCN?)>c(OH?)>c(H+)��2�֣��������Ʒ֣�
��Na2CO3 + HClO = NaHCO3 +NaClO��3�֣�
��ac��2�֣�
2CH4(g)+3O2(g)=2CO(g)+4H2O(g) ?H=��1214.0kJ?mol?1��3�֣�
��2����4�֣���0.025��2�֣��� > ��2�֣�
��3����7�֣��� c(K+)>C(SCN?)>c(OH?)>c(H+)��2�֣��������Ʒ֣�
��Na2CO3 + HClO = NaHCO3 +NaClO��3�֣�
��ac��2�֣�
�����������1��CH4ȼ����������CO2�����Կ��Ʒ�Ӧֻ����CO(g)������д��CH4��O2��Ӧ����CO��H2O�Ļ�ѧ����ʽ����ע��״̬��Ȼ����ݸ�˹��������ʱ䣺?H=��?H1+2?H2=��1214.0kJ?mol?1��������д���Ȼ�ѧ����ʽ��
��2���ٷ�Ӧ�ﵽƽ��״̬Bʱ��N2��ת����Ϊ20%����������ʽ���м���
N2(g)+3H2(g)
 2NH3(g)
2NH3(g)��ʼ���ʵ�����mol�� 10 26 0
ת�����ʵ�����mol�� 2 6 4
ƽ�����ʵ�����mol�� 8 20 4
��ƽ�ⳣ��Ϊ��K=(0.4mol?L?1)2��[0.8mol/L��(2mol?L?1)3]=0.025 L2��mol-1��
��B��N2ת���ʴ���C�㣬˵��B��ƽ�ⳣ������C��ƽ�ⳣ����A����B���¶���ͬ����ƽ�ⳣ����ͬ������K(A) > K(C)
��3����KSCNΪ����ǿ���Σ�SCN?ˮ��ʹ��Һ�ʼ��ԣ���������Ũ���ɴ�С��˳��Ϊ��c(K+)>C(SCN?)>c(OH?)>c(H+)
����ΪK1(H2CO3)>K(HClO)>K2(H2CO3)������HClO��Na2CO3��Ӧ����NaClO��NaHCO3����ѧ����ʽΪ��Na2CO3 + HClO = NaHCO3 +NaClO
��a����pH�ƿ��Բ���0��1 mol��L-1NaClO��Һ��pH�������pH>7��˵��NaClOˮ���Լ��ԣ���֤��HClOΪ���ᣬ��ȷ��b����ΪHClO����Ư���ԣ�����pH��ֽ����0��01 mol��L-1HClO��Һ��pH������c����ΪHClO������Ũ����ͬ�����������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ���ᣬ��ȷ��

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
�����Ŀ
 CH3OCH3(g)��3H2O(g)
CH3OCH3(g)��3H2O(g)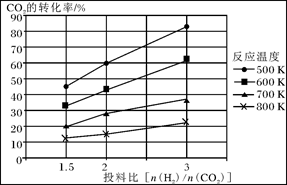
 2HgO(s)��H����181.6 kJ��mol��1
2HgO(s)��H����181.6 kJ��mol��1 CO2(g)����H1<0��
CO2(g)����H1<0�� 2NH3�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(����)
2NH3�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(����)
 2NH3(l)����H=2(a-b-c)kJ��mol-1
2NH3(l)����H=2(a-b-c)kJ��mol-1 ��CO2ռ
��CO2ռ ����C(s)��
����C(s)�� O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)��
O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)�� CH3OH��g����
CH3OH��g���� O2��g��=H2O��g������H����241.8 kJ/mol��
O2��g��=H2O��g������H����241.8 kJ/mol��
 HOCH2��CH2OH��g����2CH3OH��g�� ��H����34kJ��mol
HOCH2��CH2OH��g����2CH3OH��g�� ��H����34kJ��mol