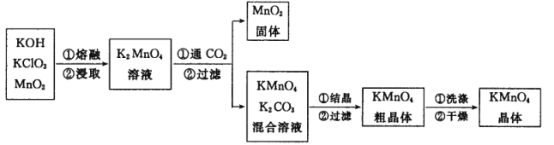
��Ŀ����
����Ŀ��(1)��֪��25 �桢101 kPaʱ��C(s)��1/2O2(g)=CO(g)����H1����110.5 kJ/mol
C(s)��O2(g)=CO2(g)����H2����393.5 kJ/mol
�Իش��������⣺
��̼��ȼ�����ǣ�__________(���H1����H2��)��
��CO(g)��1/2O2(g)=CO2(g)����H��__________kJ/mol��
(2)��25 ��ʱ����0.2 mol NO2����2 L���ܱ������У�������Ӧ��2NO2(g)![]() N2O4(g)����H����56.9 kJ/mol��5���Ӧ�ﵽƽ�⣬���NO2�����ʵ���Ϊ0.1 mol���Իش��������⣺
N2O4(g)����H����56.9 kJ/mol��5���Ӧ�ﵽƽ�⣬���NO2�����ʵ���Ϊ0.1 mol���Իش��������⣺
��5���ڣ�v(NO2)��__________mol/(L��s)��
���������������ڱ�ˮ�У�������ɫ��________(��������dz�����䡱)��
�۸÷�Ӧ��ƽ�ⳣ������ʽK��________________��
(3)NaHSO3����ѧ��ѧ���������ʡ�HSO3-��ˮ��Һ�д�����������ƽ�⣺
HSO3-=H����SO32-��Ka2
HSO3-��H2O=H2SO3��OH����Kh2
��֪25 ��ʱ��Ka2>Kh2����0.1 mol/L NaHSO3��Һ��
����Һ��__________(����ԡ������ԡ������ԡ�)��
����Һ��c(Na��)______c(HSO3-)(�>����<������)��
(4)�������������ñ���ʳ��ˮ����һ�£�������ͼ��ʾ�ľ�֧�Թ��С�
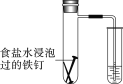
�ټ����Ӻɹ۲쵽�����е�ˮ��________��
A������ B������
��ˮ���仯��ԭ�������������˵绯ѧ��ʴ�е�________��
A�����ⸯʴ B��������ʴ
�۸õ绯ѧ��ʴ��������ӦʽΪ__________________________________��
���𰸡���H2 283 0.01 ��dz 10 ���� > A B O2��4e����2H2O=4OH��
��������
��1����ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������
���ɸ�˹���ɼ��㣻
��2�����ɻ�ѧ��Ӧ���ʹ�ʽ���㣻
�ڽ����¶ȣ�ƽ������ȵ�����Ӧ�����ƶ���
����ƽ��Ũ�Ⱥͻ�ѧƽ�ⳣ����ʽ���㣻
(4)�������������ñ���ʳ��ˮ����һ�£������֧�Թ��У���������������ʴ��
��1����ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������̼��ȼ�����Ǧ�H2���ʴ�Ϊ����H2��
�ڽ���֪�Ȼ�ѧ����ʽ���α��Ϊ1��2���ɸ�˹���ɿ�֪2��1���Ȼ�ѧ����ʽCO(g)��1/2O2(g)=CO2(g)����H������110.5 kJ/mol��������393.5 kJ/mol��=283kJ/mol���ʴ�Ϊ��283��
��2����5���ڣ���c(NO2)Ϊ��0.1 mol/L��0.05mol/L��=0.05mol/L��v(NO2)= ��c(NO2)/ ��t=0.05mol/L/5s=0.01 mol/(L��s)���ʴ�Ϊ��0.01 mol/(L��s)��
�ڽ����¶ȣ�ƽ������ȵ�����Ӧ�����ƶ����������������ڱ�ˮ�У�ƽ�������ƶ���c(NO2)��С����ɫ��dz���ʴ�Ϊ����dz��
��ƽ��ʱ��c(NO2)Ϊ0.05mol/L��c(N2O4)Ϊ0.025mol/L����Ӧ��ƽ�ⳣ������ʽK= c(N2O4)/ c2(NO2)= 0.025mol/L/(0.05mol/L)2=10���ʴ�Ϊ��10��
��25 ��ʱ��Ka2>Kh2��˵���ĵ������Һ�ʵĴ��ڢ���Һ�ʵ�ˮ�⣬��Һ�����ԣ�
��0.1 mol/L NaHSO3��Һ��Na����ˮ�⣬HSO3-�����ŵ������ƺ�ˮ�����ƣ�����c(Na��) ��c(HSO3-)���ʴ�Ϊ������
(4) �ٽ������������ñ���ʳ��ˮ����һ�£������֧�Թ��У���������������ʴ���Թ����������ʵ�����С��ѹǿ��С�����Լ����Ӻɹ۲쵽�����е�ˮ�����ߣ��ʴ�Ϊ�����ߣ�
��ˮ���仯��ԭ��������������������ʴ���ʴ�Ϊ��������ʴ��
����������������ʴʱ�������������ŵ��������������缫��ӦʽΪO2��4e����2H2O=4OH�����ʴ�Ϊ��O2��4e����2H2O=4OH����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�