��Ŀ����
����Ŀ��ij�ֽ�Ѫѹҩ��������ƽ�ĺϳ�·�����£�

��֪��
 +R����CHO
+R����CHO
(1)A �Ľṹ��ʽ�� ___________��
(2)B������Cu(OH)2 ��Ӧ�Ļ�ѧ����ʽ�� ______________��
(3)H �� I�ķ�Ӧ������ _______________��
(4)�Լ�a �� _______________��
(5)J��K�Ļ�ѧ��Ӧ����ʽ�� ___________��
(6)F �ж���ͬ���칹�壬д����������������һ��ͬ���칹��Ľṹ��ʽ__________��
�ٷ����к����������Ҵ���˳���칹
���������Na���ʷ�Ӧ�����ܷ���������Ӧ
(7)1 mol ������ƽ������__________mol NaOH��Һ����ˮ�ⷴӦ��
(8)��֪ D![]() E��F +CH3OH��E�Ľṹ��ʽ��_______________��
E��F +CH3OH��E�Ľṹ��ʽ��_______________��
���𰸡�CH��CH CH3CHO + 2 Cu(OH)2 + NaOH��CH3COONa + Cu2O��+ 3H2O ȡ����Ӧ NaOH ˮ��Һ 2 +O2
+O2![]() 2
2 +2H2O
+2H2O ![]() ��
��![]() ��
�� ��
�� 2
2 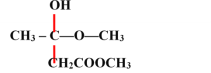
��������
AΪ��Ȳ���ṹ��ʽΪCH��CH������B��C�ķ�Ӧ������֪�÷�ӦΪȩ����������Ӧ�����A�Ľṹ��֪BΪCH3CHO����CΪCH3COOH��C��DΪ������Ӧ������DΪCH3COOCH3��������Ŀ������Ϣ�����������ƽ�Ľṹ�ɷ��Ƴ��ϳ�������ƽ������Ϊ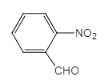 ��
��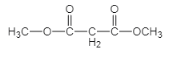 ������F��K�ĺϳ�·�߿�֪FΪ
������F��K�ĺϳ�·�߿�֪FΪ ��KΪ
��KΪ ��J��KΪ�ǻ��Ĵ�����������JΪ
��J��KΪ�ǻ��Ĵ�����������JΪ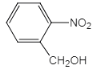 ,H��I�ķ�Ӧ����Ϊ�������տ�֪H��IΪ�����ȡ����Ӧ������HΪ
,H��I�ķ�Ӧ����Ϊ�������տ�֪H��IΪ�����ȡ����Ӧ������HΪ ,IΪ
,IΪ ����GΪ
����GΪ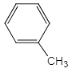 ���ݴ˽��н��
���ݴ˽��н��
��1��AΪ��Ȳ���ṹ��ʽΪCH��CH���ʴ�Ϊ��CH��CH��
��2�����ݷ�����֪BΪCH3CHO��������Cu(OH)2 ����������Ӧ���ʴ�Ϊ��CH3CHO + 2 Cu(OH)2 + NaOH��CH3COONa + Cu2O��+ 3H2O��
��3�����ݷ�����֪H��IΪ����ڹ���������������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��4��I��JΪ�ȴ�����ȡ����Ӧ����Ӧ����ΪNaOH ˮ��Һ���ȣ��ʴ�Ϊ��NaOH ˮ��Һ��
��5��J��KΪ�ǻ��Ĵ��������ʴ�Ϊ��2 +O2
+O2![]() 2
2 +2H2O��
+2H2O��
��6��F�Ľṹ��ʽΪ ����ͬ���칹�����㣺�����к�������������˳���칹˵������̼̼˫����ͬһ̼ԭ������������ͬ�Ļ��ţ��������Na���ʷ�Ӧ˵�������ǻ����Ȼ����ܷ���������Ӧ˵������
����ͬ���칹�����㣺�����к�������������˳���칹˵������̼̼˫����ͬһ̼ԭ������������ͬ�Ļ��ţ��������Na���ʷ�Ӧ˵�������ǻ����Ȼ����ܷ���������Ӧ˵������![]() �ṹ�������������ͬ���칹���У�
�ṹ�������������ͬ���칹���У�![]() ��
��![]() ��
�� 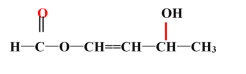 ��
�� ��
��
��7��һ��������ƽ�����к�����������������1 mol ������ƽ������2mol NaOH��Һ����ˮ�ⷴӦ���ʴ�Ϊ��2��
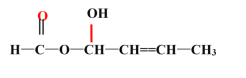
��8�����ݷ�����֪FΪ ��DΪCH3COOCH3��D��EΪ�ӳɷ�Ӧ��D��ֻ��̼��˫�����Լӳɣ��ٽ��F�Ľṹ��֪E�Ľṹ��ʽΪ��
��DΪCH3COOCH3��D��EΪ�ӳɷ�Ӧ��D��ֻ��̼��˫�����Լӳɣ��ٽ��F�Ľṹ��֪E�Ľṹ��ʽΪ��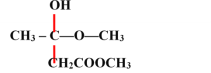 ��
��