��Ŀ����
����Ŀ����1����֪25�棺
H2S | һԪ��HA |
Ka1=9.1��10��8��Ka2=1.1��10��12�� | Ka=9.1��10��10 |
д��H2S�����KA��Һ ( A-��ʾ��� ) ��Ӧ�Ļ�ѧ����ʽ��______________________��
��2�������ᣨH3PO2����һ�ֻ�����Ʒ�����н�ǿ�Ļ�ԭ�ԡ�
��H3PO2��һԪ���ᣬд������뷽��ʽ____________________ ��
��H3PO2��NaH2PO2���ɽ���Һ�е�Ag+��ԭΪAg���Ӷ������ڻ�ѧ������NaH2PO2Ϊ________���� �����Ρ�����ʽ�Ρ���������Һ��______��������ԡ��������ԡ��������ԡ���������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4:1������������Ϊ_______���ѧʽ����
��3����NH4Al��SO4��2��Һ�еμ�1molL��1NaOH��Һ�����������ʵ��������NaOH��Һ����ı仯��ͼ��ʾ�����μӹ���������ų���
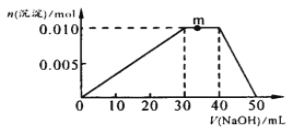
��д��m�㷢����Ӧ�����ӷ���ʽ_____________________________________��
����NH4Al��SO4��2��Һ�иļ�20mL1.2mol/L Ba��OH��2��Һ����ַ�Ӧ����Һ�в������������ʵ���Ϊ_____________mol ��
���𰸡�H2S��KA=KHS��HA H3PO2![]() H+��H2PO2- ���� ������ H3PO4 NH4+��OH-=NH3H2O 0.02
H+��H2PO2- ���� ������ H3PO4 NH4+��OH-=NH3H2O 0.02
��������
����ǿ��������ԭ�������볣��������Ʊ����볣��С����������ȫ�к����ɵ���Ϊ���Σ���ʽ��Ϊ����ʱ���ɵ������ӳ��������ӣ���NH4+����������ӵ��Σ���NH4Al��SO4��2��Һ�еμ�1molL-1NaOH��Һʱ���������ȳ������ڳ���֮��笠��������������������������ڹ����ļ�����ȫ�ܽ⡣
��1�������֪�������һ������̶ȴ���HA�ĵ���̶��ҵڶ�������̶�С��HA�ĵ���̶ȣ�����ǿ����������ɣ�H2S�����KA��Һ��Ӧ�Ļ�ѧ����ʽH2S��KA=KHS��HA��
��2����H3PO2��һԪ���ᣬ���뷽��ʽH3PO2![]() H+��H2PO2����
H+��H2PO2����
��H3PO2ΪһԪ���ᣬ������������ȫ�кͲ���NaH2PO2����NaH2PO2Ϊ���Σ�������Ϊǿ�������Σ�����Һ�������ԣ�
����H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4:1��ת�Ƶ���4mol����ӦΪH3PO2 +4Ag+= 4Ag��+ H3PO4������������ΪH3PO4��
��3����NH4Al��SO4��2��Һ�еμ�1molL-1NaOH��Һ��0~30mLʱ����ӦΪAl3++3OH-=Al(OH)3����30~40mLʱ�����������䣬��Ӧ��ҪΪNH4+��OH��=NH3H2O��40~50mLʱ�����������������ܽ⣬Al(OH)3+OH��=AlO2��+2H2O��
��д��m�㷢����Ӧ�����ӷ���ʽΪNH4+��OH��==NH3H2O��
�ڷ�Ӧ����0.01mol��������n(Al3+)=0.01mol�� 10mL����������Һ��笠����ӷ�Ӧ����n(NH4+)=0.01mol�������������ӵ���غ㣬n(SO42-)=0.02mol��
��NH4Al��SO4��2��Һ�иļ�20mL1.2mol/L Ba��OH��2��Һ����ַ�ӦBa2++ SO42-=BaSO4���������Ũ�Ȳ��㣬Ҫ��SO42-Ϊ������BaSO4����������Sԭ���غ�ò������������ʵ���Ϊ0.02mol ��