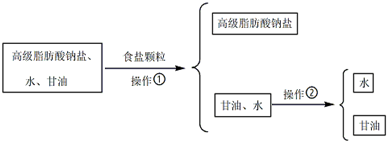
��Ŀ����
9����ù����һ�ֳ�����ҩ�ʵ�鷢����������ӽṹ�к����ǻ������п�ζ����������������̼��ĩ�˵��ǻ��е���ԭ�ӻ�������������е�ԭ����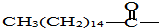 ��ζ����ʧ����Ϊ���ڿڷ�����ζ��ù�أ����¹�����ζ��ù�ص���������ȷ���ǣ�������
��ζ����ʧ����Ϊ���ڿڷ�����ζ��ù�أ����¹�����ζ��ù�ص���������ȷ���ǣ�������| A�� | ��ζ��ù�ص�ˮ���Ա����Կ�ζ��ʧ | |
| B�� | ��ζ��ù��ʧȥ��ҩ�ԣ����Կ�ζ��ʧ | |
| C�� | ��ζ��ù�������������� | |
| D�� | ��ζ��ù���������ڲ��ᷢ��ˮ�� |
���� ������̼��ĩ���ǻ���-OH���е���ԭ�ӻ�������������е�ԭ����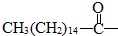 ������ζ��ù�غ�����������-COOC-�������������ˮ�����������
������ζ��ù�غ�����������-COOC-�������������ˮ�����������
��� �⣺A��-OH��Ϊ-COOC-���ܽ��Ա���ζ��ʧ����A��ȷ��
B������A֪��-OH��Ϊ-COOC-���ܽ��Ա���ζ��ʧ����B����
C����ζ��ù�غ�����������-COOC-�������������ʣ���C����
D�����������������ڷ���ˮ������������գ���D����
��ѡA��
���� ���⿼���л���ṹ�����ʣ�Ϊ��Ƶ���㣬���ؿ����������ʣ�������Ϣ�õ����ʵĽṹΪ���Ĺؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
8��A��B��C��D��EΪԭ��������������Ķ���������Ԫ�أ�A��C��B��D�ֱ�ͬ���壬��A��CԪ�ص�������֮����B��DԪ��������֮�͵�һ�룬�����жϲ���ȷ���ǣ�������
| A�� | ԭ�Ӱ뾶��С˳��C��D��B��A | |
| B�� | ��B��CԪ����ɵĻ�������ԼȺ������Ӽ����ֺ����ۼ� | |
| C�� | Ԫ��B��D��E�ֱ���A�γɵĻ������У��۷е���ߵ���B��A�γɵĻ����� | |
| D�� | Ԫ��D��C�γɵĻ������ڿ����г��ڷ��ò��ױ��� |
17��25��ʱ�������йص������Һ�����Ĺ�ϵ��ȷ���ǣ�������
| A�� | pH=4��NaHSO3��Һ�У�c��SO32-����c��H2SO3�� | |
| B�� | pH=3���Ȼ����Һ��pH=3��������ˮ�ĵ���̶���ͬ | |
| C�� | ��pH=5�Ĵ�����Һϡ�ͺ���������Ũ�Ⱦ���С��pH���� | |
| D�� | ��NH4HSO4��Һ�м�������ʵ�����NaOH�γɵ���Һ�У�c��Na+��=c��SO42-����c��NH4+����c��H+����c��OH-�� |
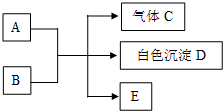
4�������£����и���������ָ����Һ���ܴ���������ǣ�������
| A�� | c��H+��=0.1mol/L����Һ��Na+��NH4+��SO42-��S2O32- | |
| B�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��10-12����Һ��K+��AlO2-��CO32-��Na+ | |
| C�� | ����ˮ�������c��H+��=1��10-12mol/L����Һ��Fe3+��ClO-��Na+��SO42- | |
| D�� | c��Fe2+��=0.1mol/L����Һ��H+��Al3+��NO3-��SCN- |
18���ɶ����ڷǽ���Ԫ��X��Ԫ��Y��ɵĻ�����Y2X3����֪X��ԭ������Ϊn����Y��ԭ�������������ǣ�������
| A�� | n-3 | B�� | n-1 | C�� | n+5 | D�� | n+2 |
 +3NaOH��3C17H35COONa+
+3NaOH��3C17H35COONa+