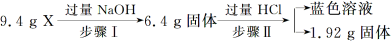��Ŀ����
�������и��������������ݣ��ɷֱ�����������ʵ������������������ʵ����ʵ���
Ũ���������жϲ���⡣
(1)��NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH��������������Һ��______________________Ϊ__________________��
(2)��֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��________Ϊ________��
(3)��֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��__________Ϊ________��
(4)��֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��________Ϊ________��
(1)���ʵ����ʵ���Ũ��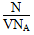 mol/L
mol/L
(2)���ʵ���������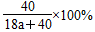
(3)���ʵ�����������44.9%
(4)���ʵ����ʵ���Ũ��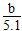 mol/L
mol/L
��������(1)n��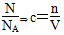 ��
��
(2)1��a?m(NaOH)��m(H2O)?w��m(NaOH)��[m(NaOH)��m(H2O)]��
(3)V(HCl)?m(HCl)?w��m(HCl)��[m(HCl)��m(H2O)]��
(4)b g Al2O3?n(AlCl3)?c��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ