��Ŀ����
����Ŀ�������ģ��״�����Ҫ�Ļ���ԭ�ϣ�CO��CO2�������ںϳɼ״�����CO2�������״��ķ�Ӧ����ʽΪ��CO2(g)+3H2(g)��CH3OH(g)+H2O(g) ��H1
��1����֪��
2CO(g)+O2(g)��2CO2(g) ��H2
2H2(g)+O2(g)��2H2O(g) ��H3
��CO(g)+ 2H2(g)��CH3OH(g) ��H4��_______
��2����CO�ϳɼ״�ʱ��CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��P���Q���ƽ�ⳣ���Ĵ�СKP____ KQ (��������������С��������������)��ʵ����������������250����1.3��104kPa���ң�ѡ���ѹǿ��������________________��
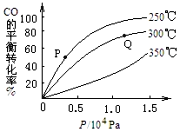
��3��һ���¶��£���2 L�ܱ������м���1 mol CH3OH(g)��������ӦCH3OH(g) ��CO(g)+ 2H2(g)��CO�����������ʱ��ı仯��ͼ��ʾ��
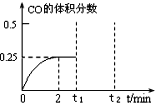
�ٷ�Ӧ�ﵽƽ��״̬�ı�־��___________��
A��������ܶȲ���
B�������ѹǿ���ֲ���
C��������CH3OH��=2����(H2)
D������(H2)=2������CO��
�ڸ��¶��£�CO(g)+ 2H2(g)��CH3OH(g)��ƽ�ⳣ��K=_________��
������t1ʱ���ټ���1mol CH3OH(g)����t2 ʱ�����´ﵽƽ�⣬����ͼ�ϻ���CO�����������ʱ��仯�����ߣ���Ҫ����������ֵ�������仯�����ƺͷ�Χ���ɣ�_______��
��4����CH3OHΪȼ��(��KOH��Һ���������Һ)���Ƴ�CH3OHȼ�ϵ�ء������ĵ缫��ӦʽΪ_________________________��
���𰸡���H1+![]() ��H2��
��H2��![]() ��H3������1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ�û��Ҫ������ѹǿʹ�����ɱ�����BD4L2��mol-2
��H3������1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ�û��Ҫ������ѹǿʹ�����ɱ�����BD4L2��mol-2 CH3OH-6e-+8OH-��CO32-+6H2O
CH3OH-6e-+8OH-��CO32-+6H2O
��������
(1)��CO2(g)+3H2(g)��CH3OH(g)+H2O(g) ��H1����2CO(g)+O2(g)��2CO2(g)��H2����2H2(g)+O2(g)��2H2O(g)��H3�����ݸ�˹���ɣ�����+![]() ����-
����-![]() ���۵ã�CO(g)+ 2H2(g)��CH3OH(g) ��H4����H1+
���۵ã�CO(g)+ 2H2(g)��CH3OH(g) ��H4����H1+![]() ��H2-
��H2-![]() ��H3���ʴ�Ϊ����H1+
��H3���ʴ�Ϊ����H1+![]() ��H2-
��H2-![]() ��H3��
��H3��
(2)�Ӻ�������һ��0.5������һ��ƽ��������������ߣ�����ͬѹǿ�²�ͬ�¶�ʱCO��ƽ��ת���ʣ��¶�Խ��ת����Խ�ͣ�˵��������ʱƽ���������ƶ������P���Q���ƽ�ⳣ���Ĵ�СKP����KQ����ҵ����Ҫ�����پ���Ч�棬Ҫ�����ٶȺ�Ч�ʣ�ѹǿԽ����Ҫ������Խ�ߣ�����Խ�ʴ�Ϊ�����ڣ���1.3��104kPa�£�CO��ת�����Ѿ��ܸߣ�û��Ҫ������ѹǿʹ�����ɱ����ӣ�
(3)��A��������������䣬������������䣬������ܶ�ʼ�ղ��䣬�����ж���ƽ��״̬���ʴ���B���÷�Ӧǰ����������ʵ��������仯�������������䣬�����ѹǿ���ֲ���ʱ��˵����������ʵ������ֲ��䣬�ܹ�˵���ﵽƽ��״̬������ȷ��C������(CH3OH)=2����(H2)��Ϊ����Ӧ���ʣ�����˵������Ӧ�������淴Ӧ������ȣ��ʴ���D������(H2)=2����(CO)��ʾ���淴Ӧ������ȣ��ﵽƽ��״̬������ȷ����ѡBD��
��CH3OH(g)CO(g)+2H2(g)
��ʼ(mol/L )0.5 0 0
��Ӧ(mol/L )0.25 0.25 0.5
ƽ��(mol/L )0.25 0.25 0.5
K=![]() =0.25(mol/L)2����CO(g)+ 2H2(g)��CH3OH(g)��ƽ�ⳣ��K=
=0.25(mol/L)2����CO(g)+ 2H2(g)��CH3OH(g)��ƽ�ⳣ��K=![]() =4 L2��mol-2��
=4 L2��mol-2��
������t1ʱ���ټ���1mol CH3OH(g)��CO���������ͻȻ��С�����ŷ�Ӧ�Ľ��У�CO������������������ټ���1mol CH3OH(g)���൱������ѹǿ�������ƶ����ﵽ��ƽ��ʱ��CO���������С��0.25��CO�����������ʱ��仯��������ͼ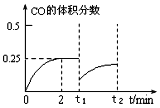 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(4)��ȼ�ϼ��Ե���У��ܷ�ӦΪ2CH3OH+3O2+4OH-=2CO32-+6H2O������ȼ�ϵĵ缫�Ǹ��������Լ���״��ĵ缫�Ǹ��������������ĵ缫��������������ӦʽΪ3O2+12e-+6H2O=12OH-���õ�ط�Ӧ����ʽ��ȥ�������õ�������ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O���ʴ�Ϊ��CH3OH+8OH--6e-=CO32-+6H2O��