��Ŀ����
����Ŀ����̬��ɢϵ��һ������ֱ������1~100nm��һ�ַ�ɢϵ����ͼ��Fe(OH)3����Ľṹʾ��ͼ������˵������ȷ���ǣ� ��
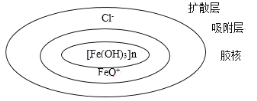
A.��ͼ�����жϣ�������Fe(OH)3�����壩+HCl![]() FeOCl+2H2O�ķ�Ӧ
FeOCl+2H2O�ķ�Ӧ
B.����������Ӿʵ��ʱ�������ܵ�Һ�����������
C.��Ӿʵ����ֵ������ԭ���ǣ���ɢ������������룬������Ľ����������ƶ�
D.���������һ��ʱ�䣬�ᷢ������ľ۳�����
���𰸡�B
��������
A����ͼ�����жϣ�Fe(OH)3(����)�г���FeO+��Cl-�������ƶϷ�����Fe(OH)3(����)+HClFeOCl+2H2O�ķ�Ӧ����A��ȷ��
B������������Ӿʵ��ʱ���������Ӷ����ƶ����������ܺ������ܵ�Һ��ʼ����ƽ�����ᷢ���仯����B����
C����ͼ��֪����ɢ������磬����������磬��Ӿʱ�����ֵ�����������ɢ������������룬������Ľ����������ƶ�����C��ȷ��
D�����Ƚ��壬���������˶��ٶȼӿ죬���������ֱ����������ӣ�������ֱ������100nm���ᷢ������ľ۳����ã���D��ȷ��
�ʴ�ѡB��

 ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�����Ŀ������������������Һ���й���������ȷ����
��� | �� | �� | �� | �� |
pH | 11 | 11 | 3 | 3 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. �ۢ��зֱ���������Ĵ����ƾ��������Һ��pH������
B. �ڢ�����Һ�������ϣ�������Һ��c(H��)��c(OH��)
C. �ֱ��ˮϡ��10����������Һ��pH�٣��ڣ��ܣ���
D. V1L����V2L����Һ��Ϻ�����Ϻ���ҺpH��7����V1��V2
����Ŀ��úȼ���ŷŵ���������SO2��NOx,�γ����ꡢ��Ⱦ����,����NaClO2��Һ��Ϊ���ռ���ͬʱ���������������������ش���������:
(1)NaClO2�Ļ�ѧ����Ϊ__________��
(2)�ڹ��ݷ�Ӧ����ͨ�뺬��SO2��NO������,��Ӧ�¶�323 K,NaClO2��ҺŨ��Ϊ5��10-3 mol/L����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±���
���� | SO42- | SO32- | NO3- | NO2- | Cl- |
c/(mol/L) | 8.35��10-4 | 6.87��10-6 | 1.5��10-4 | 1.2��10-5 | 3.4��10-3 |
�ٳ�NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ__________________________������ѹǿ,NO��ת����________________(���ߡ������䡱���͡�)��
�������շ�Ӧ�Ľ���,���ռ���Һ��pH��____________(��������䡱��С��)��
����ʵ������֪,����Ӧ����______________��Ӧ����(����ڡ���С�ڡ�)��ԭ���dz���SO2��NO�������еij�ʼŨ�Ȳ�ͬ,��������____________��
(3)�������NaClO��Ca(ClO)2���NaClO2,Ҳ�ܵõ��Ϻõ���������Ч����
�ٴӻ�ѧƽ��ԭ������,Ca(ClO)2���NaClO���е��ŵ���______________��
��֪���з�Ӧ:
SO2(g)+2OH-(aq) =SO32-(aq)+H2O(l)����H1
ClO-(aq)+SO32-(aq)=SO42-(aq)+Cl-(aq)��H2
CaSO4(s)=Ca2+(aq)+SO42-(aq)����H3
��ӦSO2(g)+Ca2+(aq)+ClO-(aq)+2OH-(aq)=CaSO4(s)+H2O(l)+Cl-(aq)�Ħ�H=______��


