��Ŀ����
����Ŀ���������и��������������ݣ��ɷֱ�����������ʵ������������������ʵ����ʵ���Ũ���������жϲ���⡣
��1����NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH-������������Һ��______Ϊ______��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��______Ϊ______��
��3����֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��______Ϊ______��
��4����֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��______Ϊ______��
���𰸡���1��NaOH�����ʵ���Ũ��![]()
��2��NaOH����������![]()
��3��HCl���������� 44.9%
��4��AlCl3�����ʵ���Ũ��![]() mol/L
mol/L
����������1����֪��Һ���ܶȣ�ֻ��������ʵ���Ũ�ȣ�c��![]() mol/L��
mol/L��
��2��NaOH��H2O�����ʵ���֮��Ϊ1��a���������ʵ�����������w��![]() ��
��
��3����֪��Һ���ܶȣ����ܼ������ʵ���Ũ�ȣ�����������������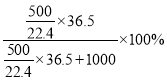
![]() ��
��
��4����ɫ����ΪAl2O3��n��Al2O3����![]() mol��n��AlCl3����
mol��n��AlCl3����![]() mol��c��AlCl3����
mol��c��AlCl3����![]() ��
��![]() mol/L��
mol/L��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij����С�����ʵ������ȡ���ᴿ���������ķ������£�
��֪�����Ȼ��ƿ����Ҵ��γ�CaCl2��6C2H5OH
���й��л���ķе㣺
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е�/�� | 34.7 | 78.5 | 118 | 77.1 |
��2CH3CH2OH![]() CH3CH2OCH2CH3��H2O
CH3CH2OCH2CH3��H2O
I���Ʊ�����
װ����ͼ��ʾ��A�з���Ũ���ᣬB�з���9.5mL��ˮ�Ҵ���6mL�����ᣬD�з��б���̼������Һ��

��1��д���������Ҵ�����������Ӧ�Ļ�ѧ����ʽ______________________________��
��2��ʵ������еμӴ�Լ3mLŨ���ᣬB���ݻ�����ʵ���________��������ȷѡ��ǰ����ĸ��
A��25mL B��50mL C��250mL D��500mL
��3�����θ���ܵ���Ҫ������_________________________��
��4������Na2CO3��Һ��������__________________________________________________________________________________________________________________________________��
II���ᴿ�������ٽ�D�л��Һת���Һ©�����з�Һ��
���л�����5mL����ʳ��ˮϴ�ӣ�����5mL�����Ȼ�����Һϴ�ӣ������ˮϴ�ӡ��л��㵹��һ�������ƿ�У�����ˮ����þ����ôֲ��
�۽��ֲ��������ռ�77.1�����֣��õ��������������������
��5���ڢٲ���Һʱ��ѡ�õ����ֲ������������Ʒֱ���__________��_______��
��6���ڢڲ����ñ���ʳ��ˮ�������Ȼ�����Һ�������ˮϴ�ӣ��ֱ���Ҫϴȥ�ֲ�Ʒ�е�________________��__________________��______________��
����Ŀ������ʯ�ң�CaO�������Na2CO3����ʳ�Σ�NaCl����һ��������Ͽ��Ƶ�һ������ԭ�ϣ�ijͬѧΪ��̽����ԭ�ϳɷ���������ʵ�飺
��ͬѧȡһ��������Ʒ����ˮ����Ҫ�����Ļ�ѧ����ʽ�У�____________________________, _____________________________________��
��2����ͬѧ��Ϊ��1�����˺����õ���Һ�п϶����д�����NaOH��NaCl���ʣ������ܺ���Ca(OH)2��Na2CO3 ��Ϊ��̽��������Һ�п��ܺ��е������Ƿ���ڣ������������ʵ�鷽����
ʵ�鷽��������±���ʾ��
ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� | �����ӷ���ʽ���� |
��ȡ������Һ���μ�����K2CO3��Һ | �����ְ�ɫ���� | ��Һ�к�Ca(OH)2 | ��:________________ |
���ް�ɫ���� | ��Һ����Ca(OH)2 |
| |
��ȡ������Һ��______________________________________________ | ��:����___________ | ��Һ�к�Na2CO3 | ��:_______________ |
��:����___________ | ��Һ����Na2CO3 |
|


