��Ŀ����
����˵�����ʾ������ȷ����(����)
| A���ⶨHCl��NaOH���к���ʱ��ÿ��ʵ���Ӧ���������¶ȣ����������ʼ�¶ȡ�NaOH����ʼ�¶Ⱥͷ�Ӧ�������ȶ����¶� |
| B��31g���ױ��31g����Ҫ����������˵�����ױȺ����ȶ� |
| C����101 kPaʱ����֪������ȼ����Ϊ285.8kJ/mol��������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ2H2(g)��O2(g)=2H2O(g)����H��-571.6 kJ/mol |
| D��2A (l) + B (l) =" 2C" (l) ��H1 2A (g) + B (g) =" 2C" (l)��H2 ��H1����H2 |
D
�������������A���ⶨHCl��NaOH���к���ʱ��ÿ��ʵ���Ӧ���������¶ȣ����������ʼ�¶ȡ�NaOH����ʼ�¶Ⱥͷ�Ӧ�����е�����¶ȡ�����B������������������Խ�����ʵ��ȶ��Ծ�Խ�Խ���ȶ�������C. ȼ������1mol��������ȫȼ�ղ����ȶ���������ʱ���ų�����������̬��ˮ�����ȶ��Ĵ���״̬������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ2H2(g)��O2(g)=2H2O(l)����H��-571.6 kJ/mol.����D.��̬���������е�������Һ̬Ҫ�࣬���Բ�����ͬ״̬�ĵ�������ͬһ����ʱ��̬���ų�������Ҫ��Һ̬�Ķࡣ�ų�������Խ�࣬��Ӧ�Ⱦ�ԽС���ʦ�H1����H2����ȷ��
���㣺�����к��ȵIJⶨ��ȼ���ȵ��Ȼ�ѧ����ʽ����д�����ʵ��ȶ��Ե�֪ʶ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ͼ���йص���������ȷ����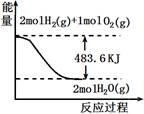
| A����ʾ��mol H2 (g)��ȫȼ������ˮ��������241.8 kJ���� |
| B����ʾ���Ȼ�ѧ����ʽΪ��H2(g)+ 1/2 O2(g) �� H2O(g) +241.8 kJ |
| C����ʾ2 mol H2(g)�����е�����һ����2 mol��̬ˮ�����е�������483.6 kJ |
| D��H2O(g)����������H2(g)��O2(g)������֮�� |
���з�Ӧ����������ԭ��Ӧ���������ȷ�Ӧ����
| A����Ƭ��ϡH2SO4��Ӧ | B��Ba(OH)2��8H2O��NH4Cl��Ӧ |
| C�����ȵ�̼��CO2��Ӧ | D������������O2�е�ȼ�շ�Ӧ |
�����й��Ȼ�ѧ����ʽ��������ȷ����
| A����֪2H2O(g) ��2H2(g)��O2(g) ��H��+483.6 kJ/mol����������ȼ����Ϊ��H����241.8kJ/mol |
| B����֪C��ʯī��s���� C�����ʯ��s����H��0������ʯ����ʯī�ȶ� |
| C����֪�к���Ϊ��H����57��4kJ/mol����ϡ�����ϡNaOH��Һ��Ӧ���Ȼ�ѧ����ʽΪ��NaOH(aq)��CH3COOH(aq)��CH3COONa(aq)��H2O(l) ��H����57.4kJ/mol |
| D����֪2C(s)��2O2(g)��2CO2 (g) ��H1��2C(s)��O2 (g)��2CO(g) ��H2�����H1����H2 |
�����к��Ȳⶨʵ�������˵������ȷ����
| A���ձ�����������ĭ������Ϊ�˼���ʵ������е�������ʧ |
| B��ʹ�û��β������ȿ��Խ����ֱ������¶ȼ� |
| C����ʢװ����ձ��мӼ�ʱҪС�Ļ��� |
| D�����������¶ȼ�Ҫ��ˮ��ϴ���ٲ����¶� |
��֪��2H2(g)��O2(g)��2H2O(l) ��H����571.6 kJ��mol��1 ��
2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1 ��
H��(aq)��OH��(aq)��H2O(l) ��H����57.3 kJ��mol��1 ����˵����ȷ���ǣ� ��
| A��H2(g)��ȼ����Ϊ571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C��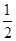 H2SO4(aq)�� H2SO4(aq)��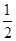 Ba(OH)2(aq)�� Ba(OH)2(aq)�� 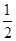 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����
| A����֪��2H2(g)��O2(g)=2H2O(g)��H����483.6 kJ��mol��1����������ȼ����Ϊ241��8 kJ��mol��1 |
| B����֪C(ʯī��s)=C(���ʯ��s)����H��0������ʯ��ʯī�ȶ� |
| C����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)����H����57.3 kJ��mol��1����20.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.65kJ������ |
| D����֪2C(s)��2O2(g)=2CO2(g)����H1, 2C(s)��O2(g)=2CO(g)����H2����H1����H2 |
ij��Ӧ�ķ�Ӧ�����������仯��ͼ��ʾ(ͼ��E��ʾ���)������������ȷ����( )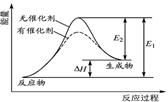
| A���淴Ӧ�Ļ�ܴ�������Ӧ�Ļ�� |
| B���÷�ӦΪ���ȷ�Ӧ |
| C�������ܸı䷴Ӧ���ʱ� |
| D�������ܽ��ͷ�Ӧ�Ļ�� |
����˵����ȷ���ǣ� ��
| A����Ӧ�Ⱦ��Ƿ�Ӧ�зų������� |
| B�����ȷ�Ӧ�ġ�H>0�����ȷ�Ӧ�ġ�H<0 |
| C��һ����ѧ��Ӧ�У�����Ӧ��������������������������ʱ����Ӧ���ȣ���HΪ������ |
| D��һ����ѧ��Ӧ�У���������ܼ��ܴ��ڷ�Ӧ����ܼ���ʱ����Ӧ���ȣ���HΪ��+�� |