��Ŀ����
5����ҵ�ϴӷ�Ǧ���ص�Ǧ�����Ǧ�Ĺ����У�����̼������Һ��Ǧ�ࣨ��Ҫ�ɷ�ΪPbSO4��������Ӧ��PbSO4��s��+CO32-��aq��?PbCO3��s��+SO42-��aq����ij��ѧ��ȤС����Ǧ��ԭ��ģ��ù��̻���Ǧ���乤���������£�
Ǧ��$\stackrel{ˮϴ}{��}$ $\underset{\stackrel{N{a}_{2}C{O}_{3}}{��}}{����}$����A$\stackrel{����}{��}$PbO$\underset{\stackrel{��̿}{��}}{����¯}$Pb
�Իش��������⣺
��1��ˮϴ��Ŀ���dz�ȥǦ�������ᣮ
��2��ʵ��������ȳ���Aʹ��ֽ⣬���˱�Ҫ����Դ�����ż����⣬����Ҫ�������������������ǡ�����ǯ�ȣ�
��3����֪�ڼ�����Һ�У���NaClO���ܽ�+2��Ǧ�Ļ�����������PbO2��������������ӷ���ʽ
Pb��OH��3-+ClO-=PbO2+Cl-+H2O+OH-��
��4��������Aʵ��Ϊ��ʽ̼��Ǧ������ˮ������ԭ�������ӷ���ʽ��Ҫ������ԭ��CO32-�ܷ���ˮ�⣬ʹ��Һ�ʼ��ԣ����Եõ���ʽ̼��Ǧ����Ӧ�����ӷ���ʽΪCO32-+PbSO4+2H2O=Pb2��OH��2CO3+SO42-+2H+��
��5����֪һ�����ij���A������ȫ�ֽ�����6.69g��PbO��ͬʱ�ռ���448mL��CO2���ѻ���ɱ�״�����������A�Ļ�ѧʽΪPb3��OH��2��CO3��2��
���� Ǧ�ࣨ��Ҫ�ɷ�ΪPbSO4����ˮϴ����ȥ������ᣬ�ּ���̼���ƣ�Ǧ����PbSO4��Na2CO3��Һ��Ӧת��ΪPbCO3����������Һ������AΪPbCO3��PbCO3���ȷֽ�õ�PbO��ͬʱ�ų�������̼���壬��̼��ԭPbO��Pb��
��1��Ǧ���������ᣬҪ��ˮϴ��
��2��������ȷֽ�Ӧ���������н��У�
��3���ڼ�����Һ�У���NaClO���ܽ�+2��Ǧ�Ļ�����������PbO2��ClO-����ԭ��Cl-�����ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��4��CO32-�ܷ���ˮ�⣬ʹ��Һ�ʼ��ԣ�ʹ����Pb2+����Pb��OH��2�������õ���ʽ̼��Ǧ��
��5������A������ȫ�ֽ�����6.69g��PbO�������ʵ���Ϊ0.03mol��ͬʱ�ռ���448mL��CO2�������ʵ���Ϊ0.02mol������n��Pb����n��C��=3��2�����ݻ��ϼ۴�����Ϊ0ȷ�����������ӵ���Ŀ������ȷ����ѧʽ��
��� �⣺Ǧ�ࣨ��Ҫ�ɷ�ΪPbSO4����ˮϴ����ȥ������ᣬ�ּ���̼���ƣ�Ǧ����PbSO4��Na2CO3��Һ��Ӧת��ΪPbCO3����������Һ������AΪPbCO3��PbCO3���ȷֽ�õ�PbO��ͬʱ�ų�������̼���壬��̼��ԭPbO��Pb��
��1��Ǧ���������ᣬҪ��ˮϴ������ˮϴ��Ŀ���dz�ȥǦ�������ᣬ
�ʴ�Ϊ����ȥǦ�������
��2��������ȷֽ�Ӧ���������н��У����Լ��ȳ���Aʹ��ֽ⣬���˱�Ҫ����Դ�����ż����⣬����Ҫ�������������������ǡ�����ǯ�ȣ�
�ʴ�Ϊ�������������ǡ�����ǯ��
��3���ڼ�����Һ�У���NaClO���ܽ�+2��Ǧ�Ļ�����������PbO2��ClO-����ԭ��Cl-����Ӧ�����ӷ���ʽΪPb��OH��3-+ClO-=PbO2+Cl-+H2O+OH-��
�ʴ�Ϊ��Pb��OH��3-+ClO-=PbO2+Cl-+H2O+OH-��
��4��CO32-�ܷ���ˮ�⣬ʹ��Һ�ʼ��ԣ�ʹ����Pb2+����Pb��OH��2�������õ���ʽ̼��Ǧ����Ӧ�����ӷ���ʽΪCO32-+PbSO4+2H2O=Pb2��OH��2CO3+SO42-+2H+��
�ʴ�Ϊ��CO32-�ܷ���ˮ�⣬ʹ��Һ�ʼ��ԣ����Եõ���ʽ̼��Ǧ����Ӧ�����ӷ���ʽΪCO32-+PbSO4+2H2O=Pb2��OH��2CO3+SO42-+2H+��
��5������A������ȫ�ֽ�����6.69g��PbO�������ʵ���Ϊ0.03mol��ͬʱ�ռ���448mL��CO2�������ʵ���Ϊ0.02mol������n��Pb����n��C��=3��2�����ݻ��ϼ۴�����Ϊ0��֪������A��n��Pb2+����n��CO32-����n��OH-���T3��2��2������A�Ļ�ѧʽΪPb3��OH��2��CO3��2 ��
�ʴ�Ϊ��Pb3��OH��2��CO3��2��
���� ���⿼����ۺϣ��漰�����ķ�����ᴿ��������ԭ��Ӧ�����ӷ���ʽ����ѧ�����֪ʶ�㣬�ܴ������Ϸ�����������ȡԭ������Ŀ�Ѷ��еȣ�

| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl-��OH-��CO32-��SO42- |
��B��Һ�ֱ���C��D��ϣ����а�ɫ�������ɣ�
�ڽ�A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��
��A��D���ֹ����ϼ������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ������
����ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ�����壮
��1��A�����������ӵĵ���ʽ��
 ��B��������������Ba2+��
��B��������������Ba2+����2��C�Ļ�ѧʽ��Al2��SO4��3��D�Ļ�ѧʽ�ǣ�NH4��2CO3��
��3��д�����г����ܽ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��NaClO��Ӧ�����Ϻ�ɫ����������Һ |
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ�������κ�KNO2�Ȳ��� |
��2�����������ˮ�м�������ɱ�������ܾ�ˮ����һ�������ˮ����������������ɱ������Ϊ���������ǿ�����ԣ����ܾ�ˮ��ԭ���Ǹ��������ˮ��Ӧ���ɵ�Fe��OH��3�����������ԣ�
��3����ʵ��������Fe��NO3��3��Һʱ����������ˮֱ�����ã�������Һ�����ϻ��ǣ���ԭ���ǣ������ӷ���ʽ��ʾ��Fe3++3H2O?Fe��OH��3+3H+����ˣ���ȷ�����ò���������Һ�м��������ģ����Լ����ƣ����ᣬ��Ŀ�������������ӵ�ˮ�⣮
��4��Fe2O3��һ�ֺ���ɫ�������д���������ᷴӦ�����ӷ���ʽFe2O3+6H+=3H2O+2Fe3+��
��5������Fe3+ �ķ����ܶ࣬д�����е�һ�ּ��鷽��ȡ������Һ���Թ��У���������KSCN��Һ��������Ѫ��ɫ��Һ������Fe3+��
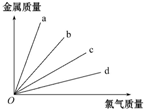 ��ͼ��ʾ���ơ�þ���������ֱ��������������Ӧʱ�����Ľ����������뷴Ӧ������������֮��Ĺ�ϵ�����б�ʾ����������Ӧ���ǣ�������
��ͼ��ʾ���ơ�þ���������ֱ��������������Ӧʱ�����Ľ����������뷴Ӧ������������֮��Ĺ�ϵ�����б�ʾ����������Ӧ���ǣ�������| A�� | a | B�� | b | C�� | c | D�� | d |
| A�� | T��ʱ��KW=1.0��10-12�����¶��£�PH=3����Һ�У�ˮ�������c��H+��=1.0��10-11 | |
| B�� | 25��ʱ��PH=a�İ�ˮ��PH=b�����������������Һ�����ԣ���a+b=14 | |
| C�� | 0.1mol/LNaHCO3��Һ�У�c��HCO3-����c��CO32-����c��H2CO3�� | |
| D�� | 0.1mol/LCH3COOH��Һ��0.05mol/LNaOH��Һ�������ϣ�������Һ�У�2c��H+��+c��CH3COOH��=c��CH3COO-��+2c��OH-�� |
 ����ѪҺ��Ca2+��Ũ��һ�����mg/cm3����ʾ����ȡһ�������Ѫ�����������IJ����[��NH4��2C2O4]��Һ������������ƣ�CaC2O4�����������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò��ᣨH2C2O4��������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�
����ѪҺ��Ca2+��Ũ��һ�����mg/cm3����ʾ����ȡһ�������Ѫ�����������IJ����[��NH4��2C2O4]��Һ������������ƣ�CaC2O4�����������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò��ᣨH2C2O4��������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2+��Ũ�ȣ�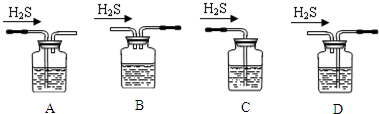
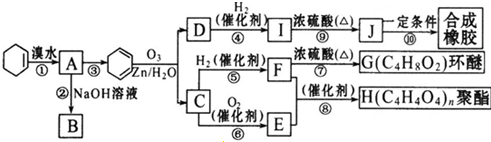 ��
��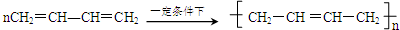 ��
��