��Ŀ����
1���������ӷ���ʽ���Ȼ�ѧ����ʽ��ȷ���ǣ�������| A�� | �ڲ�ͬ�¶��£�����ͬŨ����ͬ�����Na2S2O3��H2SO4��Ӧ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죺3S2O32-+2SO42+10H+�T6SO2��+2S��+5H2O | |
| B�� | ���������£�KI��O2���������ӷ���ʽ��4I-+O2+4H+�T2I2+2H2O | |
| C�� | ��֪25�桢101kPaʱ��1molH2����������ȫ��Ӧ������̬HBr�ų�����Q kJ�����Ȼ�ѧ����ʽΪH2��g��+Br2��g���T2HBr��g����H=-2Q kJ•mol-1 | |
| D�� | ��ʾ�к��ȵ��Ȼ�ѧ����ʽ��2HCl��aq��+Ba��OH��2��aq���TBaCl2��aq��+2H2O��l����H=-114.6 |
���� A����Ӧ���������ơ������������ˮ��
B�������������������ɵ��ˮ��
C.1molH2����������ȫ��Ӧ������̬HBr�ų�����Q kJ��
D���ʱ���λ��
��� �⣺A���ڲ�ͬ�¶��£�����ͬŨ����ͬ�����Na2S2O3��H2SO4��Ӧ��̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬���ӷ���ʽ��S2O32-+2H+�TSO2��+S��+H2O����A����
B�����������£�KI��O2���������ӷ���ʽ�����ӷ���ʽ��4I-+O2+4H+�T2I2+2H2O����B��ȷ��
C����֪25�桢101kPaʱ��1molH2����������ȫ��Ӧ������̬HBr�ų�����Q kJ�����Ȼ�ѧ����ʽΪH2��g��+Br2��g���T2HBr��g����H=-Q kJ•mol-1����C����
D����ʾ�к��ȵ��Ȼ�ѧ����ʽ�����ӷ���ʽ��2HCl��aq��+Ba��OH��2��aq���TBaCl2��aq��+2H2O��l����H=-114.6 kJ•mol-1����D����
��ѡ��B��
���� ���⿼�������ӷ���ʽ���Ȼ�ѧ����ʽ��д����ȷ��Ӧʵ�ʡ����ӷ���ʽ���Ȼ�ѧ����ʽ��д�����ǽ���ؼ���ע���ʱ䵥λ��

��ϰ��ϵ�д�
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д�
�����Ŀ
12�� ��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������
��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������
 ��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������
��ͼ����װ���У�Һ�������Ϊ200mL����ʼ����ǰ�������Һ��Ũ�Ⱦ�Ϊ0.5mol/L������һ��ʱ������0.02mol����ͨ������������Һ����ı仯������������ȷ���ǣ�������| A�� | ����������� ��=�� | |
| B�� | ���������������ӣ���������������С | |
| C�� | ��Һ��H+Ũ�ȱ仯�������ڼ�С | |
| D�� | �缫��Ӧʽ����������4OH--4e-�T2H2O+O2�������и�����2H++2e-�TH2�� |
9�������£���ָ�������У����и�������һ���ܴ���������ǣ�������
| A�� | ʹpH��ֽ�ʺ�ɫ����Һ�У�I-��Cl0-��S032-��Na+ | |
| B�� | 1.0 mol/L��KNO3��Һ�У�H+��Fe2+��Cl-��S042- | |
| C�� | ��ˮ�������C��H+��=10-13mol/L����Һ�У�C032-��S042-��Cl-��Na+ | |
| D�� | �ں��д���HC03-����Һ�У�K+��Ba2+��Cl-��N03- |
16��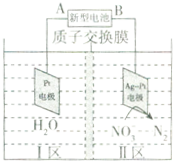 ��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������
��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������
 ��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������
��Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ�ͨ�����ԭ�����������Է�ˮ�е�NO3-������˵��������ǣ�������| A�� | �����͵�ع���ʱ�������ĵ缫��Ӧʽ��Al+4OH--3e-�TAlO2-+2H2O | |
| B�� | Ϊ��ǿ��Һ�ĵ����ԣ�I��ˮ�пɼ�������Na2SO4 | |
| C�� | AΪ��Դ������H+�Ӣ���������� | |
| D�� | ������ӦʽΪ��2NO3-+6H2O+10e-�TN2��+12OH- |
6����NA��ʾ�����ӵ�������������������ȷ���ǣ�������
| A�� | ���ʵ���Ũ��Ϊ0.5mol•L-1��MgCl2��Һ�����е�Cl-��Ϊ1NA | |
| B�� | ���³�ѹ�£�80gSO3���е���ԭ����Ϊ3NA | |
| C�� | ���³�ѹ�£�22.4LH2������ԭ����Ϊ2NA | |
| D�� | ��״���£�22.4Lˮ�������ķ�����ΪNA |
13������˵����ȷ���ǣ�������
| A�� | KClO3��SO3����ˮ���ܵ��磬��KClO3��SO3Ϊ����� | |
| B�� | HClO�����ᣬ����NaClO��������� | |
| C�� | HCl��Һ��NaCl��Һ��ͨ�����ӵ��磬����HCl��NaCl�������ӻ����� | |
| D�� | BaSO4 ��NaHCO3����ǿ����� |
 �ұ�
�ұ� 2��3-��������
2��3-��������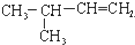 3-��-1-��ϩ
3-��-1-��ϩ ��
��