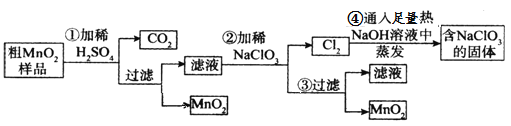
��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣��ش��й����⡣
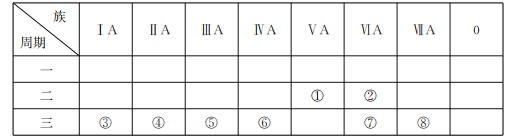
(1)д��Ԫ�ط��ţ���___����___��
(2)����ЩԪ���У�����õĽ���Ԫ�ص�ԭ�ӽṹʾ��ͼΪ___��
(3)����ЩԪ�ص�����������Ӧ��ˮ�����У�������ǿ�������������ǿ��������ˮ��Һ�з�����Ӧ�����ӷ���ʽΪ___��
(4)�ڢڡ��ۡ��ܡ�����ЩԪ���γɵ���������У����Ӱ뾶�ɴ�С��˳��Ϊ___(�����ӷ���)��
(5)��Ԫ�آڡ��ۡ����γɵ�һ�ֳ�����ɱ���������д��ڵĻ�ѧ����___��
(6)��Ԫ�آߺ͢��γɵ�ij�ֻ�����ɱ�ʾΪX2Y2(���и�ԭ�Ӿ�����8�����ȶ��ṹ)��д��X2Y2�ĵ���ʽ��___��
���𰸡�N Si ![]() H++OH-=H2O O2->Na+>Mg2+>A13+ ���Ӽ����ۼ�(�����Ӽ��ͼ��Լ�)
H++OH-=H2O O2->Na+>Mg2+>A13+ ���Ӽ����ۼ�(�����Ӽ��ͼ��Լ�) ![]()
��������
����Ԫ�������ڱ��е�λ�ÿ�֪����ΪNԪ�أ���ΪOԪ�أ���ΪNaԪ�أ���ΪMgԪ�أ���ΪAlԪ�أ���ΪSiԪ�أ���ΪSԪ�أ���ΪClԪ�أ��ݴ˽��Ԫ�ؼ��仯��������ʽ��з������
(1)��������������֪����ΪNԪ�أ���ΪSiԪ�أ��ʴ�Ϊ��N��Si��
(2)����Ԫ���У�����õĽ���Ԫ����Na����ԭ�ӽṹʾ��ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)����ЩԪ�ص�����������Ӧ��ˮ�����У�������ǿ��������HClO4��������ǿ��������NaOH��������ˮ��Һ�з�Ӧ�����ӷ���ʽΪH++OH-=H2O���ʴ�Ϊ��H++OH-=H2O��
(4)O2-��Na+��Mg2+��Al3+�ĵ��Ӳ�����Ϊ2�㣬���Ӳ�����ͬ���˵����Խ�뾶ԽС���˵����Al��Mg��Na��O�������Ӱ뾶O2-��Na+��Mg2+��A13+���ʴ�Ϊ��O2-��Na+��Mg2+��A13+��
(5)��O��Na��Cl�γɵ�һ�ֳ�����ɱ��������ΪNaClO���û�����Ϊ���ӻ�������к������Ӽ����ۼ����ʴ�Ϊ�����Ӽ����ۼ�(�����Ӽ��ͼ��Լ�)��
(6)��S��Cl��ɵĻ�����ΪS2Cl2��Ϊ���ۻ���������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�