��Ŀ����
����Ŀ��K2Cr2O7��һ����Ҫ�Ļ���ԭ�ϡ��Ը�����(��Ҫ�ɷ�ΪFeO��Cr2O3��������Al2O3��Fe2O3������)Ϊԭ���Ʊ�K2Cr2O7��һ�ֹ����������£�

��֪��
��4FeO��Cr2O3+8Na2CO3+7O2 ![]() 8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2
��Cr2O72-+H2O![]() 2CrO42-+2H+
2CrO42-+2H+
��1����������ʱ��Al2O3��Na2CO3������Ӧ�Ļ�ѧ����ʽΪ___________��
��2��������������Ҫ�ɷ���________(�ѧʽ).
��3�����ữ�����������ҺpH<5����Ŀ����_________
��4����ת����һ��������Ӧ�Ļ�ѧ����ʽΪ____________
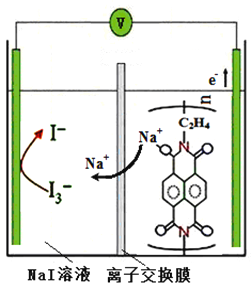
��5�����õ�ⷨ����������ˮԭ������ͼ��ʾ����������Һ�з�����Ӧ��Cr2O72-+6Fe2++14H+ == 2Cr3+ +6Fe3+ + 7H2O�������ĵ缫��ӦʽΪ_______����������������Ϊ_______(�ѧʽ)��
���𰸡� Na2CO3+Al2O3![]() 2NaAlO2+CO2�� Al(OH)3 ʹCrO42-ת��ΪCr2O72- Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl Fe-2e��=Fe2+ H2
2NaAlO2+CO2�� Al(OH)3 ʹCrO42-ת��ΪCr2O72- Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl Fe-2e��=Fe2+ H2
����������1�����յ�ʱ����Ϊ������Ϊ���������������̼���Ʒ�Ӧ�õ�ƫ�����ƣ�����ʽΪ��Na2CO3+Al2O3![]() 2NaAlO2+CO2����
2NaAlO2+CO2����
��2������������pH=7��Ŀ���ǽ���Һ��ƫ���������ת��Ϊ��������������ȥ��������������Ҫ�ɷ�ΪAl(OH)3��
��3��������֪�ڵõ���������������ҺpH<5����Ŀ���ǽ���Һ�е�CrO42-ת��ΪCr2O72-��
��4��ת����һ��������KCl��Ŀ���ǽ�Na2Cr2O7ת��ΪK2Cr2O7���������Է���ʽΪ��Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl��
��5�������缫�������壬���������缫Ҳ�ǵ���������������ӦӦ����Fe-2e��=Fe2+�����ɵ�Fe2+�ٽ�Cr2O72-��ԭΪCr3+����������Һ�е������ӣ�H+���õ��ӣ�����������������������ΪH2��

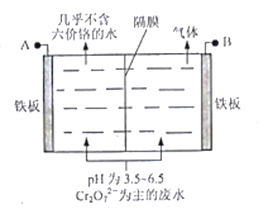
����Ŀ������ˮ�������ӵIJⶨ�����彡������Ҫ���壬ʵ���ҳ���Ī�����ⶨˮ���������Ӻ�����ʵ��������£���ˮ���м���K2CrO4 (��ɫ) ��Һ��ָʾ������AgNO3��Һ�ζ����յ㡣��֪��Ag2CrO4Ϊ������ˮ��ש��ɫ������������Ksp(AgCl)=1.8��10-10��Ksp(Ag2CrO4)= 1.8��10-12��
�ش��������⣺
��1���ζ�ʱ��Ӧʹ��____________(���ʽ����ʽ��) �ζ��ܣ�ԭ����_______________________��
��2��ˮ��Ӧ�������Ի������ԣ�ǿ����ʱ����K2CrO4��Һ������Ӧ�����ӷ���ʽ��___________________________________________��
��3���ζ��ﵽ�յ�ı�־��___________________________________________��
��4��ʵ������в���������±���
��� | 1 | 2 | 3 |
V(ˮ��)/mL | 10.00 | 10.00 | 10.00 |
c(AgNO3)/ mol��L-1 | 0.0010 | ||
V(AgNO3)/mL | 3.75 | 4.01 | 3.99 |
����ˮ���������ӵĺ���Ϊ_______________mg/L������2λС����
��5���������
�ٵ���Һ�еIJ���c(Cl-)=1.8��10-5mol/L�����ʱ��Һ��c(CrO42-)=____________��
����֪2AgCl+ CrO42-![]() 2Cl-+Ag2CrO4��������÷�Ӧ��ƽ�ⳣ��Ϊ____________��
2Cl-+Ag2CrO4��������÷�Ӧ��ƽ�ⳣ��Ϊ____________��
��6��������������ʵ��ⶨ���ƫ�͵���____________�������ţ�
A.��ƿϴ�Ӻ�δ����
B.�ζ�ǰ��δʹ�ñ�Һ��ϴ�ζ���
C.��ʽ�ζ��ܵζ�ǰ���Ӷ������ζ����Ӷ���
D.��ʽ�ζ��ܵζ�ǰ���첿�ֳ�����Һ���ζ�����ʱ�ζ��ܼ���������