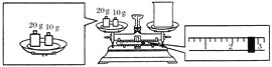
��Ŀ����
����Ŀ������ˮ�������ӵIJⶨ�����彡������Ҫ���壬ʵ���ҳ���Ī�����ⶨˮ���������Ӻ�����ʵ��������£���ˮ���м���K2CrO4 (��ɫ) ��Һ��ָʾ������AgNO3��Һ�ζ����յ㡣��֪��Ag2CrO4Ϊ������ˮ��ש��ɫ������������Ksp(AgCl)=1.8��10-10��Ksp(Ag2CrO4)= 1.8��10-12��
�ش��������⣺
��1���ζ�ʱ��Ӧʹ��____________(���ʽ����ʽ��) �ζ��ܣ�ԭ����_______________________��
��2��ˮ��Ӧ�������Ի������ԣ�ǿ����ʱ����K2CrO4��Һ������Ӧ�����ӷ���ʽ��___________________________________________��
��3���ζ��ﵽ�յ�ı�־��___________________________________________��
��4��ʵ������в���������±���
��� | 1 | 2 | 3 |
V(ˮ��)/mL | 10.00 | 10.00 | 10.00 |
c(AgNO3)/ mol��L-1 | 0.0010 | ||
V(AgNO3)/mL | 3.75 | 4.01 | 3.99 |
����ˮ���������ӵĺ���Ϊ_______________mg/L������2λС����
��5���������
�ٵ���Һ�еIJ���c(Cl-)=1.8��10-5mol/L�����ʱ��Һ��c(CrO42-)=____________��
����֪2AgCl+ CrO42-![]() 2Cl-+Ag2CrO4��������÷�Ӧ��ƽ�ⳣ��Ϊ____________��
2Cl-+Ag2CrO4��������÷�Ӧ��ƽ�ⳣ��Ϊ____________��
��6��������������ʵ��ⶨ���ƫ�͵���____________�������ţ�
A.��ƿϴ�Ӻ�δ����
B.�ζ�ǰ��δʹ�ñ�Һ��ϴ�ζ���
C.��ʽ�ζ��ܵζ�ǰ���Ӷ������ζ����Ӷ���
D.��ʽ�ζ��ܵζ�ǰ���첿�ֳ�����Һ���ζ�����ʱ�ζ��ܼ���������
���𰸡� ��ʽ AgNO3��Һˮ������� CrO 42-+2H+![]() Cr2O72-+H2O �μ����һ��AgNO3��Һʱ����ɫ���������ǣ�ǡ�ñ��ש��ɫ���Ұ���Ӳ����ԭɫ 14.20 1.8��10-2mol/L 1.8��10-8 CD
Cr2O72-+H2O �μ����һ��AgNO3��Һʱ����ɫ���������ǣ�ǡ�ñ��ש��ɫ���Ұ���Ӳ����ԭɫ 14.20 1.8��10-2mol/L 1.8��10-8 CD
����������1��AgNO3Ϊǿ�������Σ�ˮ��ʹ��Һ�����ԣ�����ʢ������ʽ�ζ����С��ʴ�Ϊ����ʽ����������Һˮ������ԡ�
��2��K2CrO4��H+�ᷢ�����淴Ӧ��CrO 42-+2H+![]() Cr2O72-+H2O������ˮ��Ӧ�������Ի������ԡ�
Cr2O72-+H2O������ˮ��Ӧ�������Ի������ԡ�
��3����Cl-ǡ�ó�����ȫʱ���ٵμ�һ����������Һ��Ag+ ��CrO 42-����Ag2CrO4��������ɫ�������ש��ɫ��������30s�ڲ���ɫ���ʴ�Ϊ���μ����һ��AgNO3��Һʱ����ɫ���������ǣ�ǡ�ñ��ש��ɫ���Ұ���Ӳ����ԭɫ��
��4����1����������Һ������������2��͵�3����ȣ����ϴ���ȥ��1�����ݣ��õ�2��͵�3�����ݵ�ƽ��ֵ���㡣���ĵ���������Һ�������ƽ��ֵΪ��3.99+4.01����10-3L/2=4��10-3L��n(Cl-)=n(Ag+)=4��10-3L��0.001 mol/L=4��10-6mol��m(Cl-)=4��10-6��35.5��103mg=142��10-3mg��ˮ���������ӵĺ���Ϊ142��10-3mg����10��10-3L��=14.20 mg/L��
��5����Ag+Ũ����ȣ�����c(Cl-)��c(Ag +)=1.8��10-10��c(CrO42-)��c2(Ag +)= 1.8��10-12 �����c(CrO42-)=1.8��10-2mol/L�� �ڶ���2AgCl+ CrO42-![]() 2Cl-+Ag2CrO4��ƽ�ⳣ��K=c2(Cl-)/c(CrO42-)= c2(Cl-)��c2(Ag +)/c(CrO42-)��c2(Ag +)=[ Ksp(AgCl)]2/ Ksp(Ag2CrO4)= (1.8��10-10)2/��1.8��10-12��=1.8��10-10��
2Cl-+Ag2CrO4��ƽ�ⳣ��K=c2(Cl-)/c(CrO42-)= c2(Cl-)��c2(Ag +)/c(CrO42-)��c2(Ag +)=[ Ksp(AgCl)]2/ Ksp(Ag2CrO4)= (1.8��10-10)2/��1.8��10-12��=1.8��10-10��
��6��.��ƿϴ�Ӻ���Ҫ����Բⶨ�����Ӱ�죻�ζ�ǰ��δʹ�ñ�Һ��ϴ�ζ��ܣ���ҺŨ�ȱ�С�������ı�Һ���ƫ���ƫ����ʽ�ζ��ܵζ�ǰ���Ӷ������ζ����Ӷ��������¶���ƫС�����ƫ�ͣ���ʽ�ζ��ܵζ�ǰ���첿�ֳ�����Һ���ζ�����ʱ�ζ��ܼ��������ݣ��в���Һ��δ�ų������ı�Һ���ƫС�����ƫ�͡��������ʵ��ⶨ���ƫ�͵���CD��

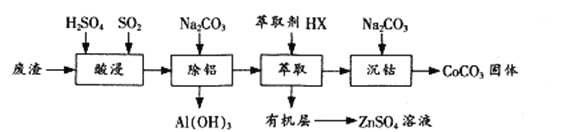
����Ŀ�����ɽ������ķ�Ӧ�ѳ�Ϊ��Ҫ�ĺϳɹ��ߣ�������ɱ��ߣ���Ի�����ɵIJ���Ӱ�졣����о��ɱ������ĺ��̡������ܡ�����ͭ�ȴ���Ӧ�ó�Ϊ�ȵ����������Ժ��ܷ�������Ҫ�ɷ�CoO��Co2O3��������Al2O3��ZnO�����ʣ�Ϊԭ���Ʊ�CoCO3��һ�ֹ���������
�±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0 mol/L���㣩
�������� | ��ʼ������pH | ������ȫ��pH |
Co2+ | 7.6 | 9.4 |
Al3+ | 3.0 | 5.0 |
Zn2+ | 5.4 | 8.0 |
�ش��������⣺
��1���������ʱͨ��SO2��Ŀ���� ____________����Ӧ�����ӷ���ʽΪ______________��
��2������ȡ�����̿ɱ�ʾΪZnSO4��ˮ�㣩+2HX���л��㣩![]() ZnX2���л��㣩+H2SO4��ˮ�㣩�����л����ȡZnSO4��Һ�IJ����� __________________��
ZnX2���л��㣩+H2SO4��ˮ�㣩�����л����ȡZnSO4��Һ�IJ����� __________________��
��3�������ܡ�ʱNa2CO3��Һ�軺���μӵ�ԭ���� ____________��
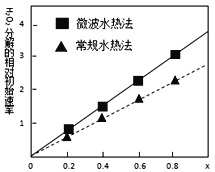
��4���õõ���CoCO3��Ϊԭ�ϲ�����ˮ�ȷ��ͳ���ˮ�ȷ������Ƶ�����CoxNi(1-x)Fe2O4������Co��Ni��Ϊ+2�ۣ�����������H2O2�ֽ�Ĵ������нϸߵĻ�������ͼ�����ֲ�ͬ�����Ƶõ�CoxNi(1-x)Fe2O4��10��ʱ���ֽ�6%��H2O2��Һ����Գ�ʼ������x�仯���ߡ���ͼ����Ϣ��֪��____________����ȡ�õ��Ĵ������Ը��ߣ��ɴ��Ʋ�Co2+��Ni2+���������д�Ч�����õ���____________ ��
��5���ô������ת����Ҳ���ԴӲ�����(CoC2O4)���ϵõ�CoCO3������CoC2O4�������Һ�еμ�Na2CO3��Һ������CoCO3��������ʱ����Һ��![]() =___________��[��֪Ksp(CoC2O4)=6.3��10-8 Ksp (CoCO3)=1.4��10- 13]
=___________��[��֪Ksp(CoC2O4)=6.3��10-8 Ksp (CoCO3)=1.4��10- 13]