��Ŀ����
����Ŀ��������Ԫ��Q��R��T��W��Ԫ�����ڱ���λ����ͼ��ʾ������T��������������������������ȣ�

��ش��������⣺
��1��T��ԭ�ӽṹʾ��ͼΪ ��R������⻯��ĵ���ʽΪ ��Q��1:1���⻯������Է���������С�ķ����� �ͷ���(���幹��)��
��2��Ԫ�صķǽ�����Ϊ(ԭ�ӵĵõ�������)��Q ___________W(����ǿ��������������)��
��3��W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ ��
��4��R�ж���������������������Է���������С����һ�������£�2 L�ļ�������0.5 L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û����������������ɵ�R�ĺ������εĻ�ѧʽ�� ��
���𰸡���1��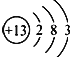 ��
��![]() ��ֱ�ߣ�
��ֱ�ߣ�
��2�����ڣ���3��S+2H2SO4![]() 3SO2��+2H2O����4��NaNO2.
3SO2��+2H2O����4��NaNO2.
�����������������T��������������������������ȣ������Ԫ�������ڱ��е����λ�ÿ�֪��TӦ���ǵ�������Ԫ�أ�����T��Al��Q��C��R��N��W��S��
��1��T����Ԫ�أ�ԭ�ӽṹʾ��ͼΪ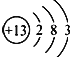 ��RΪNԪ�أ�����⻯��ĵ���ʽΪ
��RΪNԪ�أ�����⻯��ĵ���ʽΪ![]() ��QΪCԪ�أ�1:1���⻯������Է���������С�ķ�����Ȳ����ֱ���ͷ��ӣ��ʴ�Ϊ��
��QΪCԪ�أ�1:1���⻯������Է���������С�ķ�����Ȳ����ֱ���ͷ��ӣ��ʴ�Ϊ��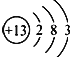 ��
��![]() ��ֱ�ߣ�
��ֱ�ߣ�
��2��QΪC��WΪS����������Դ���̼������ԣ���ǽ�����S����C���ʴ�Ϊ�����ڣ�
��3��W��SԪ�أ�������������������ˮ����Ũ��Һ���ȣ���Ӧ���������ˮ����Ӧ�Ļ�ѧ����ʽΪS+2H2SO4![]() 3SO2��+2H2O���ʴ�Ϊ��S+2H2SO4
3SO2��+2H2O���ʴ�Ϊ��S+2H2SO4![]() 3SO2��+2H2O��
3SO2��+2H2O��
��4��N�ж�����������м���Է���������С��ӦΪNO����һ�������£�2L�ļ�������0.5L���������ϣ����û�����屻������NaOH��Һ��ȫ���պ�û���������������4NO+O2+4NaOH=4NaNO2+2H2O��������ΪNaNO2���ʴ�Ϊ��NaNO2.

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�