��Ŀ����
4�� ���ж��ֻ�����ڹ�ҵ����;�㷺����������AlN���л���̼����������Ϊȷ��ij��������Ʒ�Ĵ��ȣ���������ʵ�飺����֪��AlN+H2O+NaOH��NaAlO2+NH3����
���ж��ֻ�����ڹ�ҵ����;�㷺����������AlN���л���̼����������Ϊȷ��ij��������Ʒ�Ĵ��ȣ���������ʵ�飺����֪��AlN+H2O+NaOH��NaAlO2+NH3������1����ȡ5.00g��Ʒ������25mL5mol/L��NaOH��Һ�����ȣ���Ʒ��AlN��Al2O3��ȫ��Ӧ����÷ų�����1.68L����״������ ����Ʒ�е�A1N����������Ϊ61.5%��
��2���������������Һ������Һ�еμ�2mol/L�����ᣬ������202mLʱ���ɵİ�ɫ����ǡ����ȫ��ʧ��5.00g��Ʒ��Al2O3�����ʵ����Ƕ���Ħ����д��������̣�
��ʽ̼����þ[MgxAly��OH��a��CO3��b•nH2O]����Ϊ�������ϣ�Ϊȷ��ij��ʽ̼����þ����ɣ���������ʵ�飺
��3����ȡ3.390g��Ʒ������ϡ�����ַ�Ӧ�����ɶ�����̼0.560L����״������3.390g��Ʒ�к�CO32-1.5g��
��4����ȡ100.00g��Ʒ�ڿ����м��ȣ������������¶ȵı仯��ͼ��ʾ����Ʒ��270��ʱ����ȫʧȥ�ᾧˮ��600�����Ϲ���Ϊ����������Ļ�����
��100.00g��Ʒ�к��ᾧˮ1.475mol��
��100.00g��Ʒ��OH-�����ʵ����Ƕ��٣���д��������̣�
��ͨ�������Ʋ�ü�ʽ̼����þ�Ļ�ѧʽ��
���� ��1������Ԫ���غ��֪��ˮ�μӷ�Ӧ���䷴Ӧ����ʽΪ��AlN+NaOH+H2O=NaAlO2+NH3��������NH3�����ʵ��������ݵ�Ԫ���غ��֪5g��Ʒ�к���AlN�����ʵ���������m=nM����AlN���������ٸ�����������������㣻
��2����֪���������ʵ���������ԭ���غ�õ�AlN���ɵ�NaAlO2��Al2O3Ҳת��ΪNaAlO2������NaAlO2+4HCl=NaCl+AlCl3+2H2O��NaOH+HCl=NaCl+H2O��֪4n��NaAlO2��+n��NaOH��=n��HCl���ݴ˼��㣻
��3������CԪ���غ���㣻
��4������Ʒ��270��ʱ����ȫʧȥ�ᾧˮ���ᾧˮ������=��100.00-73.45��g=26.55g������n=$\frac{m}{M}$����ˮ�����ʵ�����
��100.00g��Ʒ��270��600��֮�䣬ʧȥ�ᾧˮ�����Ʒ��һ�����ȷֽ�ų�CO2��H2O��m��CO2��+m��H2O��=73.45-37.02=36.43g���������������������100.00g�����ʷֽ����ɶ�����̼�������Ӷ�������������������ˮ���������Ӷ��������������ӵ����ʵ�����
�۸��ݵ���غ㡢�����غ����x��y���Ӷ�ȷ����ѧʽ��
��� �⣺��1������AlN������������Һ��Ӧ����NaAlO2�����ų�NH3������Ԫ���غ��֪��ˮ�μӷ�Ӧ���䷴Ӧ����ʽΪ��AlN+NaOH+H2O=NaAlO2+NH3����
����NH3�����ʵ���Ϊ$\frac{1.68L}{22.4L/mol}$=0.075mo�����ݵ�Ԫ���غ��֪5g��Ʒ�к���AlN�����ʵ���Ϊ0.075mol������Ϊ0.075mol��41g/mol=3.075g��
����Ʒ�е�AlN����������Ϊ$\frac{3.075g}{5g}$��100%=61.5%��
�ʴ�Ϊ��61.5%��
��2����֪n��NH3��=$\frac{1.68L}{22.4L/mol}$=7.5��10-2mol����AlN��֪���ɵ�n��NaAlO2��=n��NH3��������n��NaAlO2��=7.5��10-2+2n��Al2O3����
ʣ��n��NaOH��=25��5��10-3-7.5��10-2-2n��Al2O3��
�ɷ�Ӧ��NaAlO2+4HCl=NaCl+AlCl3+2H2O��NaOH+HCl=NaCl+H2O��֪��
4n��NaAlO2��+n��NaOH��=n��HCl��
��202��10-3L��2mol/L=4��[7.5��10-2+2n��Al2O3��]mol+25��5��10-3mol-7.5��10-2mol-2n��Al2O3��
��ã�n��Al2O3��=0.009mol��
��5.00g��Ʒ��Al2O3�����ʵ�����0.009mol��
��3����ȡ3.390g��Ʒ������ϡ�����ַ�Ӧ�����ɶ�����̼0.560L����״������
CO2 ��CO32-
22.4L 60g
0.56L xg
��x=$\frac{60g��0.56L}{22.4L}$=1.5g��
�ʴ�Ϊ��1.5��
��4������Ʒ��270��ʱ����ȫʧȥ�ᾧˮ���ᾧˮ������=��100.00-73.45��g=26.55g��n��H2O��=$\frac{m}{M}$=$\frac{26.55g}{18g/mol}$=1.475mol��
�ʴ�Ϊ��1.475��
��100.00g��Ʒ��270��600��֮�䣬ʧȥ�ᾧˮ�����Ʒ��һ�����ȷֽ�ų�CO2��H2O��m��CO2��+m��H2O��=73.45-37.02=36.43g��
����m��CO2��=$\frac{0.560L}{22.4L/mol}$��44g/mol��3.39g��100g=32.45g��m��H2O��=36.43-32.45=3.98g��
n��H2O��=$\frac{3.98g}{18g/mol}$=0.2212mol��
����Hԭ���غ��n��OH-��=2n��H2O��=0.4424mol��
��100.00g��Ʒ��OH-�����ʵ�����0.4424mol��
�۸��ݵ���غ��2x+3y=0.4424��1+2��$\frac{32.45}{44}$��
���������غ��40x+0.5��102y=37.02 II
����I��II��x=0.7374mol��y=0.1476mol��
x��y��a��b��n=0.7374mol��0.1476mol��0.4424mol��0.7374mol��$\frac{32.45g}{44g/mol}$��1.475mol=5��1��3��5��10��
�����仯ѧʽΪMg5Al��OH��3��CO3��5•10H2O��
�𣺼�ʽ̼����þ�Ļ�ѧʽΪMg5Al��OH��3��CO3��5•10H2O��
���� ���⿼��̽�����ʵ���ɡ���ѧ����ʽ�ļ��㣬���ؿ��������������������ȷ�������̷����ķ�Ӧ�ǽⱾ��ؼ����������ʵ����ʡ�����֮��ķ�Ӧ���������ע����ԭ���غ���з�������ȷͼ�������߱仯���Ƽ��京�壬��Ŀ�Ѷ��еȣ�


��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+�������������ӣ��йؽ��������γ������������ʱ��pH������
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
��1�����±��գ���ʵ������ѡ�����Ҫ������������
��2��д��������������Ҫ��Ӧ�����ӷ���ʽ��2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��3�����������Һ���м���ʯ�ҽ�����������ҺpH��pHӦ���ڵķ�Χ��4.7��pH��8.3��
��4����������Ҫ�ɷ�������ơ���������������������
��5����ҵ������Ϊ��ȷ����Ҫ�����Һ�м������MnO2�ۣ���ȷ��ȡ10.00mL����Һ��0.02mol/L����KMnO4��Һ�ζ����жϵζ��յ�ķ����ǵ��������һ������KMnO4��Һ����Һ���Ϻ�ɫ���Ұ�����ڲ���ɫ������ζ��յ㹲����10.00mL����KMnO4��Һ����������Һ��Fe2+Ũ����0.1mol/L��
| A�� | ����10mL | B�� | ��10mL | C�� | ����10mL | D�� | ����5mL |
| A�� | ˮ�����c��H+������=��=��=�� | |
| B�� | ���ڡ�����Һ��Ϻ�pH=7��������Һ������ۣ��� | |
| C�� | ������Ģ١��ڡ�����Һ�ֱ����������۷�Ӧ������H2��������=�ܣ��� | |
| D�� | �����Һ�м���100mLˮ����Һ��pH���ۣ��ܣ��٣��� |
| A�� | 0.01mol•L-1HA����Һ��c��H+��=1��10-4 mol•L-1 | |
| B�� | PH=3��HA��Һ��PH=11��NaOH��Һ�������Ϻ�������Һ��c��Na+����c��A-����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ���c��OH-��-c��H+����c��HA��-c��A-�� | |
| D�� | PH=3��HA��Һ��PH=11��NaOH��Һ�������1��10��Ϻ�������Һ��c��OH-��+c��A-��=c��H+��+c��Na+�� |
| A�� | ������Ľṹʽ��H-Cl-O | B�� | ������Ϊ1����ԭ�ӣ�${\;}_{1}^{1}$H | ||
| C�� | ���ĵ���ʽ��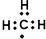 | D�� | CO2�ı���ģ�ͣ� |
