��Ŀ����
2��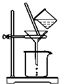 ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ�������£�
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ�������£�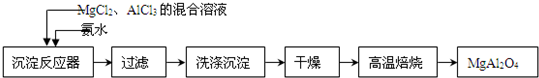
��1���Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ2Al��OH��3+Mg��OH��2$\frac{\underline{\;����\;}}{\;}$MgAl2O4+4H2O��
��2����ͼ��ʾ�����˲����е�һ��������©���¶˼���δ�����ձ��ڱڣ��ж������г����Ƿ�ϴ�����õ��Լ���AgNO3��Һ���������ữ��AgNO3��Һ�������±���ʱ������ʢ�Ź��������������������
��3����25���£���Ũ�Ⱦ�Ϊ0.01mol•L-1��MgCl2��AlCl3�����Һ����μ��백ˮ��������Al��OH��3�������ѧʽ�������ɸó��������ӷ���ʽAl3++3NH3•H2O=Al��OH��3��+3NH4+����֪25��ʱKsp[Mg��OH��2]=1.8��10-11��Ksp[Al��OH��3]=3��10-34��
��4����ˮAlCl3��183������������ʪ��������������������ʵ���ҿ�������װ���Ʊ���
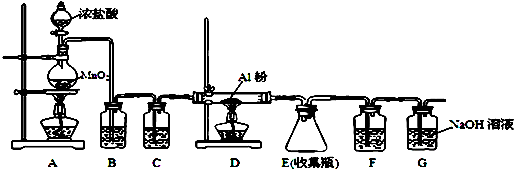
װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ�����dz�ȥHCl��F���Լ�������������ˮ��������һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ��ʯ�ң�
��5����Mg��Cu��ɵ�3.92g�����Ͷ�����ϡ�����У���ַ�Ӧ������ȫ�ܽ�ʱ�ռ�����ԭ����NO����1.792L����״��������Ӧ�����Һ�м���4mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ���������γɳ���������Ϊ8g��
���� MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ��õ�������þ�Լ����������Ļ���������������ϴ�Ӹ�����б��տ��Եõ�MgAl2O4��
��1������������ΪAl��OH��3��Mg��OH��2�����±����Ʊ�MgAl2O4ʱ������ӦΪAl��OH��3��Mg��OH��2����MgAl2O4������Ԫ���غ���д��ѧ����ʽ��
��2��Ϊ�˷�ֹҺ�彦�����ڹ���ʱӦ�ý�©���ļ��첿�ֽ����ձ����ڱڣ����������֪��������Ӧ�ø��������Ӻ�笠����ӣ����ж��Ƿ�ϴ��������ȡ�������һ��ϴ��Һ�����������ữ����������Һ�����жϣ������ɰ�ɫ��������˵��û��ϴ�Ӹɾ�����û�г������ɣ���˵���Ѿ�ϴ�Ӹɾ���
��3�����ܵ���ʵ��ܶȻ�ԽС�����백ˮʱԽ�����ɳ�������
��4������������װ��ͼ��֪��B�еı���ʳ��ˮ��Ϊ�˳�ȥ���е�HCl���壻��Ϊ�Ȼ�������ˮ�⣬��Ӧ�÷�ֹ�����е�ˮ��������Eװ�ã���G�����տ����е�CO2�����Կ��Լ����ʯ��������F��G�����ã�
��5�����ݵ��ӵ�ʧ�غ��֪������NOʱ�ĵ���ת�Ƶ����ʵ�����Ϊ����ʧȥ�ĵ��ӵ����ʵ�����Ҳ�������ɳ���ʱ��������������ӵ����ʵ���������Ԫ���غ���м��㣻
��� �⣺MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ��õ�������þ�Լ����������Ļ���������������ϴ�Ӹ�����б��տ��Եõ�MgAl2O4��
��1������������ΪAl��OH��3��Mg��OH��2�����±��ձ�MgAl2O4ʱ������Ӧ�Ļ�ѧ����ʽ2Al��OH��3+Mg��OH��2$\frac{\underline{\;����\;}}{\;}$MgAl2O4+4H2O��
�ʴ�Ϊ��2Al��OH��3+Mg��OH��2$\frac{\underline{\;����\;}}{\;}$MgAl2O4+4H2O��
��2������ʱӦ�ý�©���ļ��첿�ֽ����ձ����ڱڣ���ֹҺ�彦����������Ӧ�ø��������Ӻ�笠����ӣ����ж��Ƿ�ϴ��������ȡ�������һ��ϴ��Һ������AgNO3��Һ���������ữ��AgNO3��Һ����Һ�����жϣ������ɰ�ɫ��������˵��û��ϴ�Ӹɾ�����û�г������ɣ���˵���Ѿ�ϴ�Ӹɾ������±��չ���Ӧ�������н��У�
�ʴ�Ϊ��©���¶˼���δ�����ձ��ڱڣ�AgNO3��Һ���������ữ��AgNO3��Һ����������
��3�����ܵ���ʵ��ܶȻ�ԽС�����백ˮʱԽ�����ɳ�������֪25��ʱKsp[Mg��OH��2]=1.8��10-11��Ksp[Al��OH��3]=3��10-34�����������ȳ��������ɳ��������ӷ���ʽΪAl3++3NH3•H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ��Al��OH��3��Al3++3NH3•H2O=Al��OH��3��+3NH4+��
��4��B�еı���ʳ��ˮ��Ϊ�˳�ȥ���е�HCl���壻��Ϊ�Ȼ�������ˮ�⣬��Ӧ�÷�ֹ�����е�ˮ��������Eװ�ã���G�����շ�Ӧʣ������������Կ��Լ����ʯ��������F��G�����ã�
�ʴ�Ϊ����ȥHCl������ˮ��������ʯ�ң�
��5��NO����1.792L����״������Ϊ0.08mol������NOʱ�ĵ���ת�Ƶ����ʵ���Ϊ0.08mol��3=0.24mol����Ӧ�����Һ�м���4mol/L��NaOH��Һ80mLʱ��������ǡ����ȫ������������ϵ����������ӵ����ʵ���Ϊ0.24mol�����γɳ���������Ϊ3.92g+0.24mol��17mol/L=8g��
�ʴ�Ϊ��8��
���� ���⿼�����ʵ��Ʊ�ʵ����ƣ���Ŀ�Ѷ��еȣ�ע�����ʵ����Ƶ���������˳�����ʵ��ԭ������5���е�����Ԫ���غ���м������ѵ㣮

 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�| A�� | H2S03 | B�� | Ca��OH��2 | C�� | KCl03 | D�� | CO2 |
| A�� | CH3-CH2Cl | B�� | CH3CH=CHBr | C�� | CH3C CCH3 | D�� | CH3CH=C��CH3��2 |

| A�� | A��Ϊԭ��أ�B��Ϊ����ʳ� | |
| B�� | ʯī��C1Ϊ�������缫��ӦʽΪ2H++2e-�TH2�� | |
| C�� | ʯī��C1�����ɹ۲쵽��Һ�ʺ�ɫ | |
| D�� | ��C2������224mL���壨��״����ʱ��п�缫����������0.65g |
| A�� | 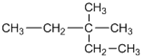 2-��-2-�һ����� 2-��-2-�һ����� | B�� | CH3C��CH3��2CH2CH3 2��2-�������� | ||
| C�� | 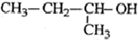 2-��-1-���� 2-��-1-���� | D�� | 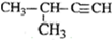 2-��-3-��Ȳ 2-��-3-��Ȳ |
| A�� | n=m+15 | B�� | n=m+5 | C�� | n=m+29 | D�� | n=m+9 |
| A�� | һ���ǽ���Ԫ�� | B�� | ������ϡ������Ԫ�� | ||
| C�� | һ�����Ƿǽ���Ԫ�� | D�� | ԭ������ʧȥ���ӵ�Ԫ�� |

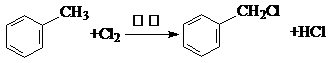 ��
��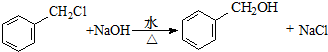 ��
�� ��������д���÷�Ӧ�Ļ�ѧ����ʽ��
��������д���÷�Ӧ�Ļ�ѧ����ʽ��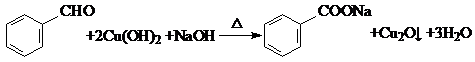 ��
��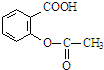 ���������ͷӣ�
���������ͷӣ�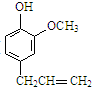 ����ҽѧ�Ͼ��й㷺��;��
����ҽѧ�Ͼ��й㷺��;��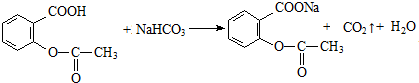 ��
��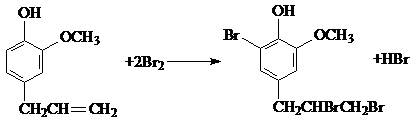 ��
�� ����д���ԶԱ������ᣨ
����д���ԶԱ������ᣨ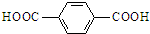 �����Ҷ���Ϊԭ�Ϻϳɵ��ڵĻ�ѧ����ʽ��
�����Ҷ���Ϊԭ�Ϻϳɵ��ڵĻ�ѧ����ʽ��