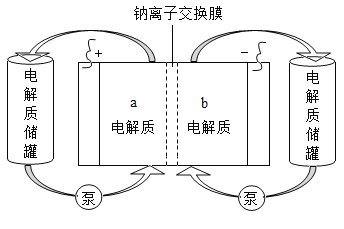
��Ŀ����
����Ŀ��(1)Na2CO3�׳ƴ��д��ˮ�ⳣ���ı���ʽΪKh=___________����֪25��ʱ��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32-) = 2��1ʱ��������Һ��pH=____��
(2)������Ũ��Ϊ0.1 mol��L-1�����ᡢ���ᡢ����������Һ��
��������Һ��Ũ������Ϊa mol��L-1��b mol��L-1��c mol��L-1�����С˳��Ϊ____________��
�ڵ������������ֱ���NaOH��Һ�к���������ʱ������NaOH�����ʵ���������n1��n2��n3�����С��ϵ_______________��
(3)��AlCl3��Һ���ɡ����գ����õ�����Ҫ���������___________________��
(4)�ڴ�����Һ�е����̪����Һ���ɫ�����ڸ���Һ���ٵ��������BaCl2��Һ�����۲쵽��������_____________��ԭ����______________�������������ӷ���ʽ�����ּ��Խ��ͣ�
���𰸡�![]() 10 c>a>b n3>n1=n2 Al2O3 ������ɫ�������Һ�ɫ��ȥ �ڴ�����Һ��CO32-ˮ�⣺CO32-+H2O
10 c>a>b n3>n1=n2 Al2O3 ������ɫ�������Һ�ɫ��ȥ �ڴ�����Һ��CO32-ˮ�⣺CO32-+H2O![]() HCO3-+OH-������BaCl2��Ba2++CO32-=BaCO3��(��ɫ)������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��
HCO3-+OH-������BaCl2��Ba2++CO32-=BaCO3��(��ɫ)������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��
��������
(1)̼������ǿ�������Σ��ܷ�������ˮ�⣬�Ե�һ��ˮ��Ϊ������һ��ˮ������̼��������Ӻ����������ӣ�ˮ�ⷽ��ʽΪ��CO32-+H2O=HCO3-+OH-��Kh=![]() ��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32-)=2��1ʱ��Kh=
��Kh=2��10-4mol/L������Һ��c(HCO3-)��c(CO32-)=2��1ʱ��Kh=![]() =2��10-4mol/L��c(OH-)=1��10-4 mol/L��c(H+)=1��10-10mol/L��������Һ��pH=10���ʴ�Ϊ��
=2��10-4mol/L��c(OH-)=1��10-4 mol/L��c(H+)=1��10-10mol/L��������Һ��pH=10���ʴ�Ϊ��![]() ��10��
��10��
(2)���Ȼ����������ǿ����ʣ�������һԪ�ᣬ�����Ƕ�Ԫ�ᣬ������һԪ���ᣬ����������������Ũ�������Ũ����ȣ�������������Ũ���������Ũ�ȵ�2����������������Ũ��С�ڴ����Ũ�ȣ����Ե�������Ũ����ͬʱ������ҺŨ�ȴ�С˳��Ϊc>a>b���ʴ�Ϊ��c>a>b��
�ڵ�������������к��е�������(����ĺ�δ�����)����c>a=b���ֱ���NaOH��Һ�к���������ʱ������NaOH�����ʵ���������n1��n2��n3�����С��ϵΪn3>n1=n2���ʴ�Ϊ��n3>n1=n2��
(3) AlCl3Ϊǿ�������Σ����ȴٽ�ˮ�������������������ᣬ�����ӷ����������������ɷֽ�����Al2O3���ʴ�Ϊ��Al2O3��
(4)������̼���ƣ�Ϊǿ�������Σ�ˮ���������������ӣ�CO32-+H2OHCO3-+OH-��������Һ�ʼ��ԣ������̪����Һ��죻����Һ�м����Ȼ��������Ӻ�̼������ӷ�Ӧ����̼�ᱵ������Ba2++CO32-�TBaCO3��(��ɫ)��CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����Һ��ɫ���ʴ�Ϊ��������ɫ�������Һ�ɫ��ȥ���ڴ�����Һ��CO32-ˮ�⣺CO32-+H2O![]() HCO3-+OH-������BaCl2��Ba2++CO32-=BaCO3��(��ɫ)������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��
HCO3-+OH-������BaCl2��Ba2++CO32-=BaCO3��(��ɫ)������CO32-Ũ�ȼ�С��ˮ��ƽ�����ƣ�OH-Ũ�ȼ�С����̪��ɫ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��25 �棬������ĵ���ƽ�ⳣ�����±�������������ȷ����(����)
Ka1 | Ka2 | |
H2A | 1.3��10-2 | 6.3��10-6 |
H2B | 4.2��10-7 | 5.6��10-11 |
A. H2A�ĵ��뷽��ʽ��H2A 2H����A2-
B. �����£���ˮ��Na2B��ˮ��ƽ�ⳣ��Ϊ��![]()
C. ��ͬpH��Na2A��Na2B��Һ�����ʵ���Ũ�ȣ�c(Na2A)��c(Na2B)
D. ��Na2B��Һ�м�������H2A��Һ���ɷ�����Ӧ��B2����H2AA2����H2B
����Ŀ��CO2����Ҫ���������壬Ҳ��һ�ֹ�ҵԭ�ϡ���������CO2�����ڻ�������ЧӦ�����Ļ������⡣
(1)�ҹ���ѧ��ͨ������һ�������ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���͡�
��֪��2H2 (g)+O2 (g) =2H2O(l) ��H = -571.6 kJ/mol
2C8H18(l)+25O2(g) =16CO2(g)+18H2O(l) ��H = -11036 kJ/mol
25�桢101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)��Һ̬ˮ���Ȼ�ѧ����ʽ��_________��
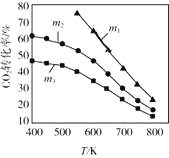
(2)CO2������ϳ��Ҵ��ķ�Ӧԭ���ǣ�2CO2(g)+6H2(g)![]() C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=
C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=![]() ��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
(3)��Cu/ZnO���������£���CO2��H2��Ͽɺϳɼ״���ͬʱ������������ƽ�з�Ӧ��
��Ӧ�� CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
��Ӧ�� CO2(g)+H2(g) ![]() CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
����һ����CO2��H2��ʼͶ�ϱȣ�����ͬѹǿ��,������ͬ��Ӧʱ��������ʵ������(�������״�ѡ��������ָת����CO2�����ɼ״��İٷֱ�)��
ʵ����� | T/K | ���� | CO2ת����/% | �״�ѡ����/% |
ʵ��1 | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
ʵ��2 | 543 | Cu/ZnO����Ƭ | 10.9 | 72.7 |
ʵ��3 | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
ʵ��4 | 553 | Cu/ZnO����Ƭ | 12.0 | 71.6 |
�ٶԱ�ʵ��1��ʵ��3�ɷ��֣�ͬ�����������£��¶����ߣ�CO2ת�������ߣ� ���״���ѡ����ȴ���ͣ�����ͼ״�ѡ���Խ��͵Ŀ���ԭ��_______________��
�ڶԱ�ʵ��1��ʵ�� 2�ɷ��֣���ͬ���¶��£�����Cu/ZnO����ƬʹCO2ת���ʽ��ͣ� ���״���ѡ����ȴ��ߣ�����ͼ״���ѡ������ߵĿ���ԭ��____________��
�����������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��_______��
a��ʹ��Cu/ZnO���װ�������
b��ʹ��Cu/ZnO����Ƭ������
c�����ͷ�Ӧ�¶�
d��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
e������![]() �ij�ʼͶ�ϱ�
�ij�ʼͶ�ϱ�
(4)������������ĤΪ�����缫��ϡ����Ϊ�������Һ����һ��������ͨ��CO2����⣬���������Ƶõ��ܶȾ���ϩ![]() (���LDPE)��
(���LDPE)��
�ٵ��ʱ�������ĵ缫��Ӧʽ��_____________��
�ڹ�ҵ������1.4��104 kg ��LDPE����������Ҫ��״����______L ��CO2��
����Ŀ��25 ��ʱ���������ʵĵ��볣�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HClO |
���볣�� | 1.7��10-5 | K1��4.3��10-7 K2��5.6��10-11 | 3.0��10-8 |
��ش��������⣺
(1)CH3COOH��H2CO3��HClO��������ǿ������˳��Ϊ______________________����д��NaHCO3��Һ�Լ��Եĵ����ӷ���ʽ________��
(2)ͬŨ�ȵ�CH3COO-��HCO3-��CO32-��ClO-���H+��������ǿ������˳��Ϊ______________��
(3)�����ͬ��pH��ͬ��CH3COOH��Һ��HClO��Һ����NaOH��Һ�к�ʱ��������NaOH�����ʵ���________(����ĸ)��
A����ͬ B���к�CH3COOH�Ķ�
C���к�HClO�Ķ� D�����Ƚ�
(4)���Ϊ10 mL pH��2�Ĵ�����Һ��һԪ��HX��Һ�ֱ��ˮ ϡ����1 000 mL��ϡ����pH�仯��ͼ��ʾ����HX�ĵ��볣�� ________(����������������������С����)����ĵ��볣����

(5)H��Ũ����ͬ�������������ҺA(����)��B(CH3COOH)�ֱ���п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����______ (��д���)��
�ٷ�Ӧ����Ҫ��ʱ��B��A���ڿ�ʼ��Ӧʱ������A��B �۲μӷ�Ӧ��п�����ʵ���A��B��
�ܷ�Ӧ���̵�ƽ������B��A����A����пʣ�ࡡ��B����пʣ��