��Ŀ����
����Ŀ������[KAl(SO4)2��12H2O]���������������й㷺��;������ˮ�ľ�������ֽ��ҵ����ʩ������ʳƷ��ҵ�ķ��ͼ��ȡ������������ķ�����������(��Al��Al2O3������SiO2��FeO��xFe2O3)���Ʊ������������������£��ش��������⣺
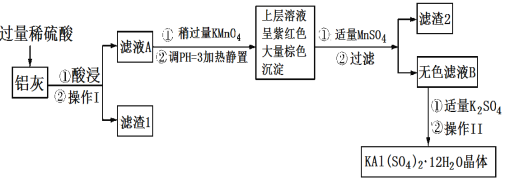
��1��������ˮ��ԭ����______________(�����ӷ���ʽ��ʾ)��
��2����������________��������������Ũ����__________�����ˡ�ϴ�ӡ����
��3��������ҺA���Ƿ����Fe2�����Լ���__________(ֻ��һ���Լ�)��
��4��������Ͷ������������Һ����������Ļ�ѧ����ʽ��__________������ҺA�м��������ط�����Ӧ�����ӷ���ʽΪ(��������MnO4-ת��ΪMn2��)��_______��
��5����֪����pH��3�����������£�MnO4-����Mn2����Ӧ����MnO2������MnSO4������Ӧ�����ӷ���ʽΪ��________������2���е�������________��
���𰸡�Al3����3H2O![]() Al(OH)3(����)��3H�� ���� ��ȴ�ᾧ ���Ը��������Һ(�����軯����Һ) 2Al��2NaOH��2H2O=2NaAlO2��3H2�� 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O 3Mn2����2MnO4-��2H2O=5MnO2����4H�� MnO2��Fe(OH)3
Al(OH)3(����)��3H�� ���� ��ȴ�ᾧ ���Ը��������Һ(�����軯����Һ) 2Al��2NaOH��2H2O=2NaAlO2��3H2�� 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O 3Mn2����2MnO4-��2H2O=5MnO2����4H�� MnO2��Fe(OH)3
��������
��1��������ǿ�������Σ�![]() ˮ����������������壬�ܹ�����ˮ�������������γɳ�������ȥ���Ӷ��ﵽ��ˮ��Ŀ�ġ��䷴Ӧԭ���÷���ʽ��ʾ��Al3����3H2O
ˮ����������������壬�ܹ�����ˮ�������������γɳ�������ȥ���Ӷ��ﵽ��ˮ��Ŀ�ġ��䷴Ӧԭ���÷���ʽ��ʾ��Al3����3H2O![]() Al(OH)3(����)��3H����
Al(OH)3(����)��3H����
��2���������ǽ������Թ�������Һ����IJ������й��ˡ�������������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����͵õ�������
�ʴ𰸣����ˣ���ȴ�ᾧ��
��3��������ҺA���Ƿ����![]() �ķ����������軯����Һ���������ɫ������֤������
�ķ����������軯����Һ���������ɫ������֤������![]() ���ʴ𰸣����軯����Һ��
���ʴ𰸣����軯����Һ��
��4��2Al��2NaOH��2H2O=2NaAlO2��3H2����Al2O3+2OH-=2AlO2-+H2O������ҺA�м��������ص�Ŀ����ʹ![]() ת��Ϊ
ת��Ϊ![]() ��������Ӧ�����ӷ���ʽΪ5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O��
��������Ӧ�����ӷ���ʽΪ5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O��
��5�����ݱ������ݿ�֪����Һ��pH=3ʱ![]() �����γɳ���
�����γɳ���![]() ��������ɵ÷���ʽ3Mn2����2MnO4-��2H2O=5MnO2����4H����������Һ��pH=3�����������ijɷֺ���MnO2��Fe(OH)3��
��������ɵ÷���ʽ3Mn2����2MnO4-��2H2O=5MnO2����4H����������Һ��pH=3�����������ijɷֺ���MnO2��Fe(OH)3��