��Ŀ����
13��ij��ѧ�о���ѧϰС�����Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ���ǣ���������pH=3��ǿ����Һ1mL����ˮϡ����100mL����ҺpH����2����λ
��1L 0.50mol•L-1NH4Cl ��Һ��2L 0.25mol•L-1 NH4Cl ��Һ��NH4+ ���ʵ���ǰ�ߴ�
����ij������Һ�к��е����ʵ�����Cl-��I-��AlO2-��CO32-��NO3-��SiO32-�������֣����������������������ݣ���Һ��ɫ�����������������3�֣���ԭ��Һ��һ����CO32-
��pH=4��Ũ�Ⱦ�Ϊ0.1mol•L-1 ��CH3COOH��CH3COONa�����Һ�У�c��CH3COO-��-c��CH3COOH��=2����10-4-10-10�� mol/L��
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
���� ��PH=-lg��c��H+�������ˮϡ�ͣ�pH���ߣ�
��NH4Cl Ũ��ԽС��NH4+ˮ��̶�Խ��
�۵����������������Һ��ɫ���˵��һ������NO3-��I-�����������������²���һ���������壬����ԭ��Һ�в�һ����CO32��
�ܳ����£����ҺpH=4��c��H+��=1.0��10-4mol/L������Kw��֪��c��OH-��=1.0��10-10mol/L���ɵ���غ��֪��c��CH3COO-��-c��Na+��=c��H+��-c��OH-�����������غ㣺2c��Na+��=c��CH3COOH��+c��CH3COO-�����ݴ˷�����
��� �⣺��PH��������Ũ�ȳɷ��ȣ����ˮϡ�ͣ�pH���ߣ��ʢٴ���
��NH4Cl Ũ��ԽС��NH4+ˮ��̶�Խ������ˮ��1L 0.50mol•L-1NH4Cl ��Һ��2L 0.25mol•L-1 NH4Cl ��Һ笠������ʵ�����ͬ����0.25mol•L-1NH4Cl ��Һ��笠�ˮ��̶ȴ����Ժ�NH4+ ���ʵ���ǰ�ߴʢ���ȷ��
��ƫ��������Ӻ������ᷴӦ���������ӣ���������Ӻ��ᷴӦ���ɹ��������NO3-��I-�����������������²���һ���������壬��������������3�֣�����ΪAlO2-��NO3-��I-����SiO32-��NO3-��I-��ԭ��Һ�в�һ����CO32-���ʢ۴���
�ܳ����£����ҺpH=4��c��H+��=1.0��10-4mol/L������Kw��֪��c��OH-��=1.0��10-10mol/L���ɵ���غ��֪����c��CH3COO-��-c��Na+��=c��H+��-c��OH-�����������غ㣺��2c��Na+��=c��CH3COOH��+c��CH3COO-�������2+��ɵã�c��CH3COO-��-c��CH3COOH��=2��c��H+��-c��OH-����=2����10-4-10-10�� mol/L���ʢ���ȷ��
��ѡD��
���� ���⿼��pH�����ϡ�͡�����ˮ�⼰���ԵıȽϡ������й���ȣ�ע��ˮ�������ԽϡԽˮ�����������ע��������Һ���������ҺpH�ļ��㷽������Ŀ�Ѷ��еȣ�

| A�� | ˮ������ | B�� | ˮ�ľ��� | C�� | ˮ������ | D�� | ˮ�ĵ�� |
��
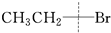
��

��
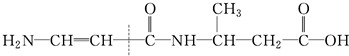
��

| A�� | �٢� | B�� | �ڢۢ� | C�� | �٢� | D�� | ȫ�� |
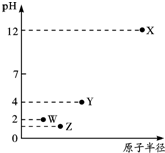
| A�� | �����Ӱ뾶��X��Y��Z��W | |
| B�� | YԪ�ش���ͬ�������� | |
| C�� | ��̬�⻯����ȶ��ԣ�Z��W��Y | |
| D�� | X��Y������������Ӧ��ˮ����ǡ���к�ʱ����Һ������ |
| A�� | ��̬�⻯����ȶ��ԣ�HX��H2Y��ZH3 | B�� | ԭ�Ӱ뾶��X��Y��Z | ||
| C�� | �ǽ����ԣ�X��Y��Z | D�� | ���������ԣ�X��Y��Z |
| A�� | �٢ܢ� | B�� | �٢ڢ� | C�� | �ڢۢ� | D�� | �ڢۢ� |
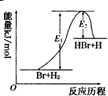
| A�� | �÷�Ӧ�ķ�Ӧ�ȡ�H=E2-E1 | |
| B�� | ����������û�ѧ��Ӧ�ķ�Ӧ�Ⱥ�ƽ�ⳣ����� | |
| C�� | ����Ӧ�����ȷ�Ӧ | |
| D�� | �����¶ȿ���������Ӧ���ʣ������淴Ӧ���� |
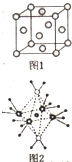 X��Y��Z��R��M��QΪǰ������Ԫ�أ���ԭ��������������YZ2�Ǻ���ɫ���壻X��YԪ�ؿ��γ�YX3��R+��Z2-������ͬ�ĺ�������Ų���M-��M���������K���ϵ��ӵ�4����Q2+���ӵ�3d�������9�����ӣ�
X��Y��Z��R��M��QΪǰ������Ԫ�أ���ԭ��������������YZ2�Ǻ���ɫ���壻X��YԪ�ؿ��γ�YX3��R+��Z2-������ͬ�ĺ�������Ų���M-��M���������K���ϵ��ӵ�4����Q2+���ӵ�3d�������9�����ӣ�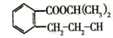 ������������ˮ������M��N��
������������ˮ������M��N��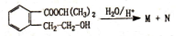
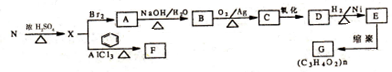
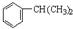 ��
��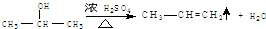 ��Eת��ΪG�Ļ�ѧ����ʽΪ
��Eת��ΪG�Ļ�ѧ����ʽΪ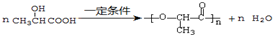 ��
��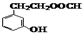 ��
��