��Ŀ����
����Ŀ��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬѧ�ֱ�������������ʵ�顣
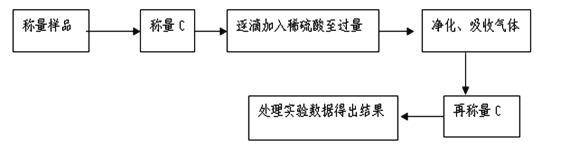
��������������������ͬѧ������ͼ��ʾ��ʵ�����̽���ʵ�飺
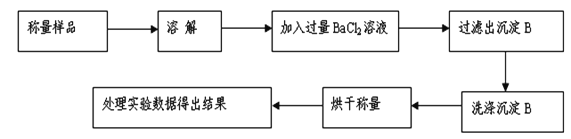
��1��ʵ��ʱ�����˲����У������ձ����������⣬��Ҫ�õ��IJ�������Ϊ___________��
��2��ϴ�ӳ���B�IJ�����__________________________________��
��3����ʵ���в����Ʒ����Ϊm g����������Ϊn g����̼���Ƶ���������Ϊ____________��
���������������������ͬѧ����Ҫʵ������ͼ���£�
������ͼ��ʾװ�ý���ʵ�飺
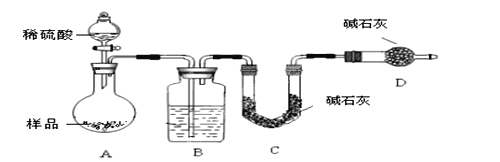
��4����ʵ����װ��Bʢ�ŵ�������_________________��
��5��Dװ�õ�������____________________________________��
��6��Ϊ����ʵ�����ڷ�Ӧǰ��ͨ��N2����Ӧ��ͨ��N2��Ŀ����_____________________��
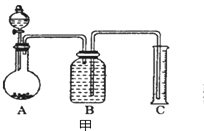
��7����һ������Ʒ������ϡ���ᷴӦ������ͼװ�ò�������CO2����������B��Һ��ò���_______������ѡ����ѡ��ʹ��������С��
a����̼������Һ���� b����̼��������Һ ��
c��������������Һ������d��������ͭ��Һ
��8����ѡ�ø���Һ��ʵ������Ȼ����ȷ����ԭ�������__________________________��
���𰸡�©���ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2-3�Ρ�106n/197m��100%Ũ�������տ����е�ˮ������CO2����ȷ��ǰһ���������������������ȷ�Խ�A,Bװ���в���CO2ȫ������Cװ�õļ�ʯ���У���Сʵ�����b����ˮ�����ڲ���������
��������
������1�����˲����У������ձ����������⣬��Ҫ�õ��IJ�������Ϊ©������ȷ�𰸣�©����
��2��ϴ�ӳ���B�IJ������ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2-3�Σ���ȷ�𰸣��ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2-3�Ρ�
��3��̼�ᱵ����Ϊn g�����ʵ���Ϊn/197mol,����̼��������غ���ɣ�n(Na2CO3)= n(BaCO3)= n/197mol,̼���Ƶ�����Ϊ106n/197g����̼���Ƶ���������Ϊ106n/197m ��100%����ȷ�𰸣�106n/197m ��100%��
������4����ʯ���������ⶨ��Ӧ�����Ķ�����̼�������������˱���ˮ��������װ��C�ڣ�����Ҫ��ȥˮ������ʵ����װ��Bʢ�ŵ�������Ũ�����ȷ�𰸣�Ũ���ᡣ
��5��װ��C�����շ�Ӧ���ɶ�����̼��Ϊ�˼�Сʵ������Ҫ��������е�ˮ������CO2����װ��C�ڣ�����Dװ�õ����������տ����е�ˮ������CO2����ȷ��ǰһ���������������������ȷ�ԣ���ȷ�𰸣����տ����е�ˮ��������ȷ��ǰһ���������������������ȷ�ԡ�
��6��A,Bװ���в���CO2��ͨ�뵪�����Ѳ�����CO2ȫ������Cװ�õļ�ʯ���У���Сʵ������ȷ�𰸣���A,Bװ���в���CO2ȫ������Cװ�õļ�ʯ���У���Сʵ����
��7��CO2��̼������Һ��Ӧ��CO2������������Һ��Ӧ��������ͭ��Һ�л�������������̼�����ܽ⣬��CO2���岻���ڱ���̼��������Һ���ܽ����С����֮Ҳ��������Ӧ������B��Һ��ò���̼��������Һ����ȷѡ��b��
��8����ѡ�ø���Һ��ʵ������Ȼ����ȷ����ԭ������Dz��������ڻ����ˮ�֣����²ⶨ���Ķ�����̼����ƫС����ȷ��������ˮ�����ڲ��������ڡ�