��Ŀ����
�������ӷ���ʽ��ȷ����
| A����̼�������Һ�м����������Ca2+��2HCO3-��2H+��CO2����2H2O��CaCO3�� |
| B����������Һ������������Һ��Fe3+��SO42����Ba2+��3OH-��Fe(OH)3����BaSO4�� |
| C�����������Һ��ͨ��������������Ca2+��ClO-��SO2��H2O��CaSO4����Cl-��2H�� |
| D������������Һ��ͨ�����������̼��OH-��CO2��HCO3- |
D
�������������A����̼�������Һ�м�����������ӦΪHCO3-��H+��CO2����H2O������B�����ӷ���ʽ��������������������������ɣ�����C��������Ca(ClO)2����ɣ�����D�������Ķ�����̼���������Ʒ�Ӧ����̼���������ȷ��
���㣺���⿼�����ӷ���ʽ��

��ϰ��ϵ�д�
�����Ŀ
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��NO2ͨ��ˮ�У�3NO2+H2O=2H++2NO3-+NO |
B��������ͭ�缫���CuSO4��Һ��2Cu2++2H2O 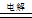 2Cu��+O2��+4H+ 2Cu��+O2��+4H+ |
| C��SO2ͨ��FeCl3��Һ�У�SO2+Fe3++2H2O=SO42-+Fe2++4H+ |
| D��������CO2ͨ��NaAlO2��Һ�У�2AlO2-+CO2+3H2O =Al(OH)3��+CO32- |
�������и������ӵ���Һ�У����������ˮ�����ܴ����������
| A��Na+��Fe2����SO42����Cl�D |
| B��Ag+��Al3+��H+��NO3�D |
| C��Na+��K+��SO42����SO32�� |
| D��NH4+��Ca2+��NO3�D��Cl�� |
��������������Һ�зֱ��������NaOH���塢����Ũ������������Ը��������Һ������ʹ����Ũ���½�����
| A��Fe3+ | B��HS�� | C��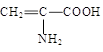 | D��C6H5O�� |
�±��е����ӷ���ʽ�����۶��������ǣ� ��
| ��� | ��ѧ��Ӧ | ���ӷ���ʽ | �� �� |
| A | ̼�������ᷴӦ | CO32-��2CH3COOH�� CO2����H2O��2CH3COO�� | ����̼�����������ʣ���Ӧд��������ʽ |
| B | NaHSO3��ˮ�� | HSO3-��H2O SO32-��H3O+ SO32-��H3O+ | ����ˮ�ⷽ��ʽ��д�ɵ��뷽��ʽ |
| C | ��������Һ��ͨ������CO2 | C6H5O����CO2��H2O�� CO 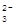 ��C6H5OH ��C6H5OH | ��ȷ |
| D | �����ʵ����� FeBr2��Cl2��Ӧ | 2Fe2+ ��2Br����2Cl2�� 2Fe3+��Br2��4Cl�� | ����Fe2+��Br�������ʵ���֮���뻯ѧʽ���� |
���и�������һ���ܴ����������
A��ij��ɫ����������Һ��Cl����Na+��MnO4-�� |
B����ʹpH��ֽ������ɫ����Һ��Na+��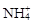 ��K+�� ��K+��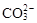 |
| C���������NaOH��Һ��ɵõ��������Һ��K+��Ba2+��HCO3-��Cl�� |
| D����ˮ���������c(H+)=10?12mol/L����Һ��K+��Ba2+��Cl����NO3�� |
�������ӷ���ʽ��ȷ����
| A�������ȥˮ���е�CaCO3��CaCO3��2H+=Ca2+��H2O��CO2�� |
| B����ˮ��ͨ��������SO2��I2 +SO2+2H2O=2I��+SO42��+4H+ |
| C��NaHSO4��Һ��Ba(OH)2��Һ��Ӧ�����ԣ�H+��SO42����Ba2+��OH��=BaSO4����H2O |
| D����ǿ����Һ�д���������Fe(OH)3��Ӧ����Na2FeO4�� |
�������ӷ���ʽ������ȷ���ǣ� ����
| A�����Ȼ�����Һ�еμ�HI��Һ��2Fe3++ 2HI = 2Fe2++2H++I2 |
B����NH4Al��SO4��2��Һ�е���Ba��OH��2ǡ��ʹSO42����Ӧ��ȫ��2Ba2++4OH��+Al3++2SO42��=2BaSO4��+AlO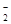 +2H2O +2H2O |
| C��1mol/L��NaAlO2��Һ��2.5 mol/L��HCl��������Ȼ�ϣ�2AlO2����5H+ = Al(OH)3��+Al3+��H2O |
| D���ù���������ữ�ĺ����ҽ���Һ����ȡ��:2I-+H2O2=I2+2OH-�� |