��Ŀ����
7����98%��ŨH2SO4����=1.84g/cm3������250ml 0.5mol/L��ϡH2SO4�IJ����ɸ���Ϊ���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���밴Ҫ����գ�
��1������ŨH2SO4�����Ϊ6.8ml��
��2�����ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ��10mL��Ͳ��ã���ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��������ҺŨ�Ƚ�ƫ�ͣ�ƫ�ߡ�ƫ�͡���Ӱ�죩��
��3������Ͳ�ͽ�ͷ�ι�����õ��IJ��������У��ձ�250ml����ƿ��������
��4��ϴ�Ӳ���һ��Ҫ���ظ�2-3�Σ���ϴ��Һ��Ҫת��������ƿ�У�
��5�����ݵIJ���Ϊ��������ƿ�м�������ˮ����Һ����̶���1-2cm�������ý�ͷ�ιܵμӣ�����Һ����ʹ���̶������У�
��6�����ƹ����У����в���������Ũ��ƫ�ߵ��Ǣڢܣ�
��δϴ���ձ��������� ��H2SO4��Һδ��ȴ�����¾�ת�Ƶ�����ƿ��
������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶�
��ҡ�Ⱥ���Һ�潵�ͣ��ټ�ˮ���̶��ߣ�
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�����ϡ���������ʵ����ʵ������������ҪŨ���������
��2��������ҪŨ�������ѡ����ʵ���Ͳ���������������ʵ����ʵ�����Ӱ�죬����C=$\frac{n}{V}$������������
��3����������һ�����ʵ���Ũ����Һ�IJ���ѡ��������
��4������ϴ�ӵIJ������
��5�����ݶ��ݵ���ȷ�������
��6���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��98%��ŨH2SO4����=1.84g/cm3�������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=1.84g/mol������ҪŨ�������ΪV����V��18.4mol/L=250mL��0.5mol/L�����V=6.8mL��
�ʴ�Ϊ��6.8mL��
��2����ȡ6.8mLŨ������Ҫѡ��10mL��Ͳ����ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��Ũ���ᱻϡ�ͣ���ȡ����������ʵ���ƫС����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��10��ƫ�ͣ�
��3������һ�����ʵ���Ũ����Һ�IJ��裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ���Ҫ����������Ͳ����������250mL����ƿ����ͷ�ιܣ������õ��IJ��������У��ձ� 250ml����ƿ ��������
�ʴ�Ϊ���ձ� 250ml����ƿ ��������
��4��ϴ�Ӳ���һ��Ҫ���ظ�2-3�Σ���ϴ��Һ��Ҫת��������ƿ�У�
�ʴ�Ϊ��2-3��ת��������ƿ�У�
��5�����ݵIJ���Ϊ��������ƿ�м�������ˮ����Һ����̶���1-2cm�����ý�ͷ�ιܵμӣ�����Һ����ʹ���̶������У�
�ʴ�Ϊ��1-2cm����Һ����ʹ���̶������У�
��6����δϴ���ձ������������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��H2SO4��Һδ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ܶ���ʱ���ӿ̶ȣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
��ҡ�Ⱥ���Һ�潵�ͣ��ټ�ˮ���̶��ߣ�������Һ�������ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ѡ���ڢܣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ���ǽ���ؼ���ע������ƿ����Ͳ����ѡ����Ŀ�ѶȲ���

| A�� | BaCl2��Һ��Na2CO3��Һ������ϡ���� | |
| B�� | Ba��OH��2��Һ��KNO3��Һ������ϡH2SO4 | |
| C�� | AgNO3��Һ��Na2CO3��Һ������ϡHCl | |
| D�� | Fe������CuSO4��Һ��Ӧ�������ϡH2SO4 |
��֪��N2��g��+2O2��g��=2NO2��g������H=+67.7kJ/mol��
N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-534kJ/mol��
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ�У���ȷ���ǣ�������
| A�� | 2N2H4��g��+2NO2��g��=3N2��g��+4H2O��l������H=-1135.7 kJ/mol | |
| B�� | 2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1000.3 kJ/mol | |
| C�� | N2H4��g��+NO2��g��=3/2N2��g��+2H2O��l������H=-1135.7 kJ/mol | |
| D�� | 2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7 kJ/mol |
| A�� | �����涣ҧ�Ժ��������������ʹƤ�����ף���ͿĨһЩϡ��ˮ�����ˮ���� | |
| B�� | �����Ƶ�Cu��OH��2�벡����Һ���ȣ��ɼ��������Ƿ��������� | |
| C�� | ����FeCl3��Һ����ij������ˮ���Ƿ��б��� | |
| D�� | ͭ�����������õĵ����ԣ����Ե繤����ʱ�ɽ�ͭ�ߺ����߽ʽ� |
| A�� | ���� | B�� | ���� | C�� | ��Һ | D�� | ���� |
| A�� | �ڱ�״���£�1mol �κ����ʵ����ԼΪ22.4L | |
| B�� | 22.4L���������ʵ���һ����1mol | |
| C�� | �ڱ�״����0.5mol �����������Ļ������������ԼΪ11.2L | |
| D�� | ���1mol��������Ϊ22.4L������Щ����һ�����ڱ�״�� |
| A�� | �������������е������Ӻ������ӿ�����������ȥ | |
| B�� | �Ȼ������������Ȼ�泥��ӹ������ռ���Һ��������� | |
| C�� | ���������л����������ᣬ�ñ���̼������Һϴ�Ӻ��Һ | |
| D�� | Ӳ֬��������л��������ĸ��ͣ�����������ȥ |
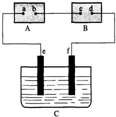 ͼ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ��CΪʢ��ϡ����ĵ��ۣ�e��fΪ���缫����ͨ��·����B�ϵ�c���Ժ�ɫ���Իش�
ͼ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ��CΪʢ��ϡ����ĵ��ۣ�e��fΪ���缫����ͨ��·����B�ϵ�c���Ժ�ɫ���Իش�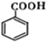 ��
��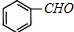 ��
��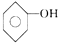 ��
��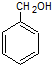 ��
��