��Ŀ����
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
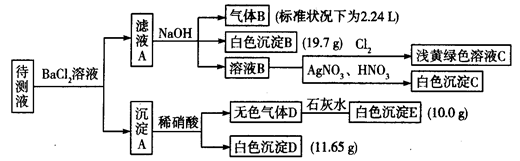
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��________(�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ________��
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��________(�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ________��
(1)n(Cl)��0.0250 L��0.40 mol��L��1��0.010 mol,0.54 g��0.010 mol��35.5 g��mol��1��0.185 g��n(Fe)�� ��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
(2)0.1��HCl��Cl2
(3)2Fe3����2I��=2Fe2����I2��(4)2Fe(OH)3��4OH����3ClO��=2FeO42-��3Cl����5H2O��
FeO42-��3e����4H2O=Fe(OH)3��5OH����
3Zn��2FeO42-��8H2O=3Zn(OH)2��2Fe(OH)3��4OH��
 ��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3(2)0.1��HCl��Cl2
(3)2Fe3����2I��=2Fe2����I2��(4)2Fe(OH)3��4OH����3ClO��=2FeO42-��3Cl����5H2O��
FeO42-��3e����4H2O=Fe(OH)3��5OH����
3Zn��2FeO42-��8H2O=3Zn(OH)2��2Fe(OH)3��4OH��
(1)n(H��)��n(OH��)��n(Cl��)��0.40 mol��L��1��25.0��10��3 L��0.010 mol,0.54 g��Ʒ��m(Cl)��0.010 mol��35.5 g��mol��1��0.355 g����m(Fe)��0.54 g��0.355 g��0.185 g��n(Fe)�� ��0.0033 mol����n(Fe)��n(Cl)��0.0033��0.010��1��3������x��3��(2)���������
��0.0033 mol����n(Fe)��n(Cl)��0.0033��0.010��1��3������x��3��(2)��������� ��
�� ��0.1����������ǿ�����ԣ��ɽ���ֱ������Ϊ��3�ۣ�������������Խ�����(3)Fe3���н�ǿ�������ԣ��ܽ�I�������ɵ��ʣ���Ӧ���ӷ���ʽΪ��2Fe3����2I��=I2��2Fe2����(4)��ǿ������Һ�У�Fe3����Fe(OH)3��ʽ���ڣ�KClO����Fe(OH)3�����ᱻ��ԭΪCl������������������д����Fe(OH)3��ClO���D��FeO42-��Cl�������ݵ�ʧ�����غ���ƽ�����������ӣ�2Fe(OH)3��3ClO���D��2FeO42-��3Cl����H2O���ٽ�ϵ���غ㼰�����Ǽ��ԵĿɵõ���2Fe(OH)3��3ClO����4OH��=2FeO42-��3Cl����5H2O��K2FeO4�������ϵõ����ӱ���ԭΪFe3����FeO42-��3e����4H2O=Fe(OH)3��5OH������ع�����пת��ΪZn(OH)2�����ܷ�ӦΪ��2FeO42-��3Zn��8H2O=2Fe(OH)3��3Zn(OH)2��4OH����
��0.1����������ǿ�����ԣ��ɽ���ֱ������Ϊ��3�ۣ�������������Խ�����(3)Fe3���н�ǿ�������ԣ��ܽ�I�������ɵ��ʣ���Ӧ���ӷ���ʽΪ��2Fe3����2I��=I2��2Fe2����(4)��ǿ������Һ�У�Fe3����Fe(OH)3��ʽ���ڣ�KClO����Fe(OH)3�����ᱻ��ԭΪCl������������������д����Fe(OH)3��ClO���D��FeO42-��Cl�������ݵ�ʧ�����غ���ƽ�����������ӣ�2Fe(OH)3��3ClO���D��2FeO42-��3Cl����H2O���ٽ�ϵ���غ㼰�����Ǽ��ԵĿɵõ���2Fe(OH)3��3ClO����4OH��=2FeO42-��3Cl����5H2O��K2FeO4�������ϵõ����ӱ���ԭΪFe3����FeO42-��3e����4H2O=Fe(OH)3��5OH������ع�����пת��ΪZn(OH)2�����ܷ�ӦΪ��2FeO42-��3Zn��8H2O=2Fe(OH)3��3Zn(OH)2��4OH����
 ��0.0033 mol����n(Fe)��n(Cl)��0.0033��0.010��1��3������x��3��(2)���������
��0.0033 mol����n(Fe)��n(Cl)��0.0033��0.010��1��3������x��3��(2)��������� ��
�� ��0.1����������ǿ�����ԣ��ɽ���ֱ������Ϊ��3�ۣ�������������Խ�����(3)Fe3���н�ǿ�������ԣ��ܽ�I�������ɵ��ʣ���Ӧ���ӷ���ʽΪ��2Fe3����2I��=I2��2Fe2����(4)��ǿ������Һ�У�Fe3����Fe(OH)3��ʽ���ڣ�KClO����Fe(OH)3�����ᱻ��ԭΪCl������������������д����Fe(OH)3��ClO���D��FeO42-��Cl�������ݵ�ʧ�����غ���ƽ�����������ӣ�2Fe(OH)3��3ClO���D��2FeO42-��3Cl����H2O���ٽ�ϵ���غ㼰�����Ǽ��ԵĿɵõ���2Fe(OH)3��3ClO����4OH��=2FeO42-��3Cl����5H2O��K2FeO4�������ϵõ����ӱ���ԭΪFe3����FeO42-��3e����4H2O=Fe(OH)3��5OH������ع�����пת��ΪZn(OH)2�����ܷ�ӦΪ��2FeO42-��3Zn��8H2O=2Fe(OH)3��3Zn(OH)2��4OH����
��0.1����������ǿ�����ԣ��ɽ���ֱ������Ϊ��3�ۣ�������������Խ�����(3)Fe3���н�ǿ�������ԣ��ܽ�I�������ɵ��ʣ���Ӧ���ӷ���ʽΪ��2Fe3����2I��=I2��2Fe2����(4)��ǿ������Һ�У�Fe3����Fe(OH)3��ʽ���ڣ�KClO����Fe(OH)3�����ᱻ��ԭΪCl������������������д����Fe(OH)3��ClO���D��FeO42-��Cl�������ݵ�ʧ�����غ���ƽ�����������ӣ�2Fe(OH)3��3ClO���D��2FeO42-��3Cl����H2O���ٽ�ϵ���غ㼰�����Ǽ��ԵĿɵõ���2Fe(OH)3��3ClO����4OH��=2FeO42-��3Cl����5H2O��K2FeO4�������ϵõ����ӱ���ԭΪFe3����FeO42-��3e����4H2O=Fe(OH)3��5OH������ع�����пת��ΪZn(OH)2�����ܷ�ӦΪ��2FeO42-��3Zn��8H2O=2Fe(OH)3��3Zn(OH)2��4OH����
��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ