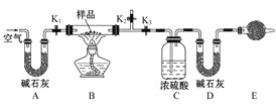
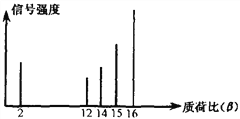
��Ŀ����
����Ŀ����һ�������£�����̼̼˫����ijЩ�л�������ɱ�ijЩ���������������ɺ���ȩ���Ļ�������磬����ϩ��![]() ����һ�������¿ɱ������ɶ���ȩ������ij�л�������A(C6H8)����һ��������������������Ӧ�����ɱ����л�������B(C6H12)������ij�������������л�������Aʱ��ֻ����һ�ֲ���C(OHC-CH2-CHO)���ش��������⣺
����һ�������¿ɱ������ɶ���ȩ������ij�л�������A(C6H8)����һ��������������������Ӧ�����ɱ����л�������B(C6H12)������ij�������������л�������Aʱ��ֻ����һ�ֲ���C(OHC-CH2-CHO)���ش��������⣺
(1)A�Ľṹ��ʽΪ___��
(2)B��C�����Ʒֱ�Ϊ___��__��
(3)д����������������A�Ļ�״ͬ���칹��Ľṹ��ʽ��___��___��___��
������̼���ϵĹ�������A��ͬ����Ŀ��ȣ�������̼���ϵ�̼ԭ������A��1��
���𰸡�![]() ������ ����ȩ
������ ����ȩ ![]()
![]()
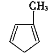
��������
����ϩ��![]() ����һ�������¿ɱ�������OHCCH2CH2CHO���Աȷ�Ӧ���������ѷ�Ӧ���е�̼̼˫���Ͽ����ٽ�˫��̼ԭ����������ԭ�ӱ�õ������������2��-CHO�ϵ�Oԭ��ȥ�������ӳ�̼̼˫������ɷ�Ӧ����ԣ��ɲ���C(OHC-CH2-CHO)�������Ƴ���6��̼ԭ�ӻ��ķ�Ӧ��Ľṹ��ʽ��
����һ�������¿ɱ�������OHCCH2CH2CHO���Աȷ�Ӧ���������ѷ�Ӧ���е�̼̼˫���Ͽ����ٽ�˫��̼ԭ����������ԭ�ӱ�õ������������2��-CHO�ϵ�Oԭ��ȥ�������ӳ�̼̼˫������ɷ�Ӧ����ԣ��ɲ���C(OHC-CH2-CHO)�������Ƴ���6��̼ԭ�ӻ��ķ�Ӧ��Ľṹ��ʽ��
(1)��2��OHC-CH2-CHO �е�4��-CHO����ȥ���γɾ���2��̼̼˫������Ԫ������ɵõ�A����A�Ľṹ��ʽΪ![]() ����Ϊ��
������![]() ��
��
(2)һ�������£�A������������Ӧ�����ɱ����л�������B(C6H12)����BΪ![]() ������Ϊ�����飬CΪOHC-CH2-CHO��������Ϊ����ȩ����Ϊ�������飻����ȩ��
������Ϊ�����飬CΪOHC-CH2-CHO��������Ϊ����ȩ����Ϊ�������飻����ȩ��
(3)����������������̼���ϵĹ�������A��ͬ����Ŀ��ȣ�������̼���ϵ�̼ԭ������A��1����A�Ļ�״ͬ���칹�壬����1����Ԫ�����ж���̼̼˫��(![]() )�������1��-CH3ȡ����������ܽṹ��ʽΪ
)�������1��-CH3ȡ����������ܽṹ��ʽΪ![]() ��
��![]() ��
��![]() ������
������![]() ��
��![]() ��
��![]() ��
��

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ����ס��ҡ������������ܱ������зֱ����һ������A��B��������Ӧ��A(g)+xB(g)![]() 2C(g)��
2C(g)��
����������±�����Ӧ������C��Ũ����ʱ��仯��ϵ����ͼ������˵����ȷ����
���� | �� | �� | �� |
�ݻ� | 0.5L | 0.5L | 1.0L |
�¶�/�� | T1 | T2 | T2 |
��Ӧ�� ��ʼ�� | 1.5 mol A 0.5 mol B | 1.5 mol A 0. 5 mol B | 6 mol A 2 mol B |
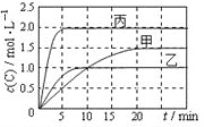
A. T1>T2��x=1
B. T2��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ0. 8
C. A��ƽ��ת����a(��)��a(��)=2��3
D. 15��20min��C��ƽ����Ӧ����v(��)< v(��)