��Ŀ����
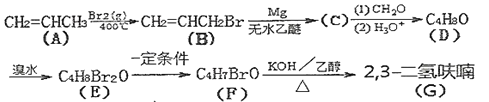
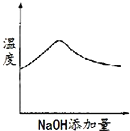
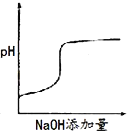
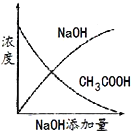
����Ŀ��ij��ɫ��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�������еļ��֣��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ������˵��һ����ȷ���ǣ� ��

A.һ������H+��Mg2+��Al3+��NH4+��һ��������Na+��SO42-��Fe3+
B.һ������H+��Al3+��NH4+��SO42-�����ܴ���Na+��Mg2+
C.��Һ��c(H+)��c(Al3+)��c(Mg2+)=1��1��2
D.��Һ��c(H+)/c(SO42-)![]() 2/9
2/9
���𰸡�D
��������
��ɫ��Һ˵������Fe3+����ͼ���֪����������������������ճ���������ȫ�ܽ⣬˵��ԭ��Һ��Mg2+��Al3+�����ʣ��1mol Mg (OH)2�����������������ֵ��2mol������Al(OH)3���������ʵ�����1mol���ܽ�1molAl(OH)3��������1�������������Һ������1�������������Һ�к���1mol�������ƣ���ʼʱ����������˵������H+��H++OH-=H2O��������������NaOH��Һ�����Ϊ1�����˵�������ӵ����ʵ���Ϊ1mol��ͼ������һ��ƽ̨��˵������OH-ʱ�������ɣ�������NH4+��NH4++ OH-=NH3H2O��笠�������������Ϊ3�����˵��笠����ӵ����ʵ�����3mol��
A.���ݷ���֪��������Һ�д���H+��Mg2+��Al3+��NH4+�����ݵ���غ㣬��һ����SO42-���������Ӳ���ȷ������A����
B.���ݷ���֪��������Һ��һ������H+��Mg2+��Al3+��NH4+��SO42-�����ܴ���Na+��һ��������Fe3+����B����
C. ���ݷ���֪������Һ�к��е�H+��Mg2+��Al3+�����ʵ�������1mol������Ũ������ȣ���c(H+)��c(Al3+)��c(Mg2+)=1��1��1����C����
D.����Һ��һ������H+��Mg2+��Al3+��NH4+��SO42-��H+��Mg2+��Al3+��NH4+�����ʵ����ֱ���1mol��1mol��1mol��3mol����Ϊ�����Ӳ�֪���Ƿ���ڣ����Ը��ݵ���غ㣬��Һ��c(H+)/c(SO42-)С�ڵ���2/9����D��ȷ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�