��Ŀ����
��9�֣������������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
(1)��ҵұ�����Ļ�ѧ����ʽ��__________________________.
(2)��������������Һ��Ӧ�����ӷ���ʽ�� .
(3)��ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ.
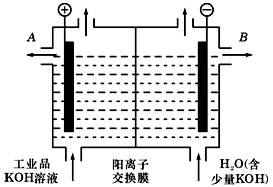
�ٸõ��۵�������Ӧʽ��___________________.
��ͨ�翪ʼ������������ҺpH�����������ԭ��__________ .
�۳�ȥ���ʺ������������Һ�ӳ���______(��д��A����B��)������
(1)��ҵұ�����Ļ�ѧ����ʽ��__________________________.
(2)��������������Һ��Ӧ�����ӷ���ʽ�� .
(3)��ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ.

�ٸõ��۵�������Ӧʽ��___________________.
��ͨ�翪ʼ������������ҺpH�����������ԭ��__________ .
�۳�ȥ���ʺ������������Һ�ӳ���______(��д��A����B��)������
��9�֣���1��2Al2O3 ===4Al+3O2��
��2��2Al+2OH��+2H2O==2AlO2��+3H2��
��2Al+2OH��+6H2O ===2[Al(OH)4]��+3H2��]
��3����4OH- -4e- = 2H2O+O2��
�����������Ϸ����缫��ӦΪ��2H++2e- = H2����ʹc(H+)��С��c(OH��)����pH���� ��B
��

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
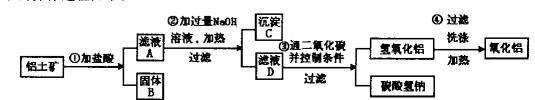
 ���
��� �ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ��
�ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ��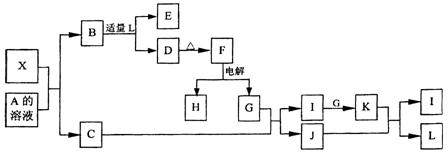
 4X��SiC��3C��
4X��SiC��3C��
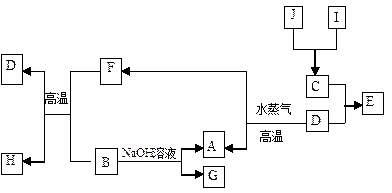
 ����ȷ���� �� ��
����ȷ���� �� ��