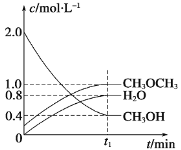
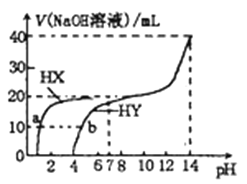
��Ŀ����
����Ŀ�������£���һԪ��HA����Һ��KOH��Һ��������(��������仯)��ʵ���������±��������жϲ���ȷ����

A. ʵ��ٷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
B. 0.1 mol��L��1HA����Һ����ˮ�������c(H��)��1��10��13 mol��L��1
C. ʵ��ڷ�Ӧ�����Һ�У�c(A��)��c(HA)��0.1 mol��L��1
D. ʵ��ڷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
���𰸡�B
��������
A��0.1 mol��L��1��HA����Һ��0.1 mol��L��1��KOH��Һ�������ϣ�ǡ����ȫ��Ӧ����KA����Ӧ����ҺpH=9��˵��KA��ǿ����������A��ˮ����B��HA��������0.1 mol��L��1HA����Һ��������������Ũ��С��0.1 mol��L��1��C����x=0.2������Һ�ʼ���������x>0.2�����ݵ���غ㣬c(K��)+ c(H��)= c(A��)+c(OH��)��pH=7��˵��c(OH��)��c(H��)������c(K��)��c(A��)��
0.1 mol��L��1��HA����Һ��0.1 mol��L��1��KOH��Һ�������ϣ�ǡ����ȫ��Ӧ����KA����Ӧ����ҺpH=9��˵��KA��ǿ����������A��ˮ��������ʵ��ٷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)����A��ȷ��HA��������0.1 mol��L��1HA����Һ��������������Ũ��С��0.1 mol��L��1��������ˮ�������c(H��)>1��10��13 mol��L��1����B����C����x=0.2������Һ�ʼ���������x>0.2�����������غ���c(A��)��c(HA)��0.1 mol��L��1����C��ȷ�����ݵ���غ㣬c(K��)+ c(H��)= c(A��)+c(OH��)��pH=7��˵��c(OH��)��c(H��)������c(K��)��c(A��)��c(K��)��c(A��)��c(OH��)��c(H��)����D��ȷ��