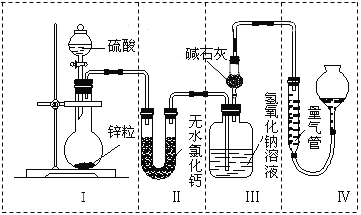
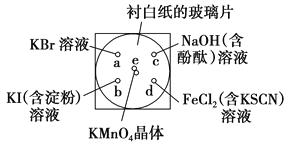
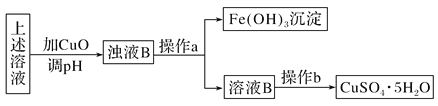
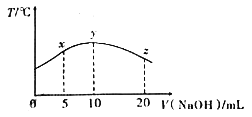
��Ŀ����
����Ŀ����Cl2����ijЩ�����л���ʱ�����������HCl�����÷�ӦA����ʵ���ȵ�ѭ�����á�
��ӦA��4HCl+O2![]() 2Cl2+2H2O
2Cl2+2H2O
��֪��I����ӦA�У�4molHCl���������ų�115.6kJ������
II��
�ж�����˵����ȷ���ǣ� ��
A. ��ӦA����H����115.6kJ��mol��1
B. H2O��H��O����HCl��H��Cl����
C. ��II�е������ж���Ԫ�صķǽ����Ա���Ԫ��ǿ
D. �Ͽ�1molH��O����Ͽ�1molH��Cl�������������31.9kJ
���𰸡�D
�����������������A����ӦA����H=-115.6kJ/mol��A����B��E(H-O)��E(HCl)�ֱ��ʾH-O���ܡ�H-Cl���ܣ���ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ����H=��Ӧ���ܼ���-��������ܼ��ܣ��ʣ�4��E(H-Cl)+498kJ/mol-[2��243kJ/mol+4��E(H-O)]=-115.6kJ/mol�������ã�4E(H-Cl)-4E(H-O)=-127.6kJ/mol����E(H-O)-E(HCl)=31.9kJ/mol��H2O��H��O����HCl��H��Cl��ǿ��B����C���ɷ�Ӧ���жϣ������ܹ���������Ϊ��������Ԫ�صķǽ����Ա���Ԫ������C����D��E(H-O)��E(HCl)�ֱ��ʾH-O���ܡ�H-Cl���ܣ���ӦA�У�4mol HCl���������ų�115.6kJ����������Ӧ����H=��Ӧ���ܼ���-��������ܼ��ܣ��ʣ�4��E(H-Cl)+498kJ/mol-[2��243kJ/mol+4��E(H-O)]=-115.6kJ/mol�������ã�4E(H-Cl)-4E(H-O)=-127.6kJ/mol����E(H-O)-E(HCl)=31.9kJ/mol���ʶϿ�1mol H-O����Ͽ�1mol H-Cl�������������ԼΪ31.9kJ/mol��1mol=31.9kJ��D��ȷ����ѡD��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�