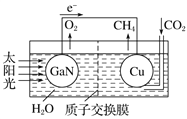
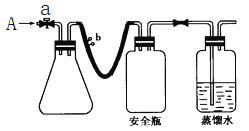
��Ŀ����
����Ŀ��ʵ���ҿ�������غ�Ũ���ᷴӦ����ȡ��������Ӧ����ʽ���£�KClO3 + 6HCl��Ũ��== KCl + 3Cl2��+ 3H2O
��1���÷�Ӧ�з�����ԭ��Ӧ��������__________������������___________��
��2������0.2mol���ӷ���ת��ʱ�����ɵ����������Ϊ________L����״��������������HCl�����ʵ���Ϊ______mol��
��3�������ɵ�����ͨ�뵽ʯ�����У�������ǡ�÷�Ӧʱ�����Ƶ�Ư��_________g��
���𰸡� KClO3 Cl2 2.688 0.2 15.24
��������������� ��KClO3 + 6HCl��Ũ��== KCl + 3Cl2��+ 3H2O���÷�Ӧ�У�������е�+5�۵���Ԫ�ػ��ϼ۽�Ϊ0��Ũ������-1����Ԫ����Ϊ0���������������������Ũ�����ǻ�ԭ�����������������������ǻ�ԭ����������ת����Ŀ��5e-���Ȼ��ؼȲ�����������Ҳ���ǻ�ԭ������Բμӷ�Ӧ��HCl�У�ֻ��![]() ��������
��������
��1���÷�Ӧ�з�����ԭ��Ӧ��������KClO3 ������������Cl2 ��
��2���ɷ�Ӧ����ʽ��֪������5mol����ת��ʱ��������3mol���������Ե���0.2mol���ӷ���ת��ʱ�����ɵ����������ʵ���n(Cl2)= ![]() ����Щ�����ڱ�״���µ����Ϊ0.12mol
����Щ�����ڱ�״���µ����Ϊ0.12mol![]() 22.4L/mol=2.688L��1mol Cl-������ΪCl2ʱʧȥ1mol���ӣ����Ա�������HCl�����ʵ������ڵ��ӵ����ʵ���������0.2mol���ӷ���ת��ʱ����������HCl�����ʵ���Ϊ0.2mol��
22.4L/mol=2.688L��1mol Cl-������ΪCl2ʱʧȥ1mol���ӣ����Ա�������HCl�����ʵ������ڵ��ӵ����ʵ���������0.2mol���ӷ���ת��ʱ����������HCl�����ʵ���Ϊ0.2mol��
��3�������ɵ�����ͨ�뵽ʯ�����У�������ǡ�÷�Ӧʱ���ɷ�Ӧ�Ļ�ѧ����ʽ2Cl2 + 2Ca(OH)2 === CaCl2 + Ca(ClO)2 + 2H2O����֪n[CaCl2Ca(ClO)2]= ![]() n(Cl2)=0.06mol�����Կ��Ƶ�Ư�۵�����Ϊ0.06mol
n(Cl2)=0.06mol�����Կ��Ƶ�Ư�۵�����Ϊ0.06mol![]() 254g/mol=15.24g��
254g/mol=15.24g��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�