��Ŀ����
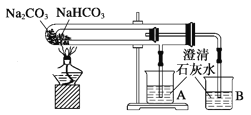
����Ŀ�����ʵķ����ж��ַ��������ж��������������ͼ��

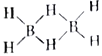
��1����ͼ��ʾ�����ʷ������������________��
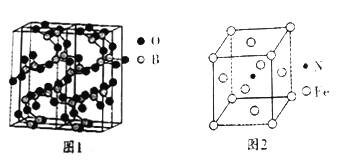
��2����Ԫ�� Na��Ba��H��O��S��N ���������ֻ�����Ԫ����ɺ��ʵ����ʣ�����ѧʽ�ֱ������±��Тܡ��ĺ���________��__________

��3���ߺ͢��ˮ��Һ�ɵ��磬����________����ǡ����ǡ�������ʣ���ͬ������������������ԭ�ӵĸ�����Ϊ________����״���µ�����������������Ϊ________��
��4���������������ܵ���Һ��Ӧ�����ӷ���ʽΪ________��
��5��д������ᷴӦ�Ļ�ѧ����ʽ________��
��6��д����ҵ������������Т�NH3 ��������Ӧ�Ļ�ѧ����ʽΪ________���� 16g ����ȫ������ԭ����ת�Ƶ���________mol��
��7�������ɢ���ڻ�ϵ�ϡ��Һ 100mL�����Тٵ����ʵ���Ũ��Ϊ 2.0mol��L��1���ڵ����ʵ���Ũ��Ϊ 1.0mol��L��1�������Һ���ܽ�ͭ���������Ϊ________g��ͬʱ����Ӧ���ɵ������ڱ�״���µ����Ϊ________L��
���𰸡���״���෨ NaOH��Ba(OH)2 BaSO4 ���� 16:11 11:16 SO2+2OH-=SO32-+H2O��Ba2++ SO2+2OH-= BaSO3��+H2O 2CO2+2Na2O2= 2Na 2CO3+O2�� 4NH3+5O2![]() 4NO+6H2O 2 9.6 2.24
4NO+6H2O 2 9.6 2.24
��������
��1����1����״���෨��һ�ֺ�����ķ��෨������һ�ô�����
��2����������������ȫ��Ϊ���������ӣ��ε������������Ϊ�������ӣ�������Ϊ������ӣ�
��3�����ݵ���ʵĶ����жϣ�����![]() ����ͬ�����Ķ�����̼����������������ԭ�ӵĸ����ȣ���״���µ����������̼��������������ʵ�����ȣ�
����ͬ�����Ķ�����̼����������������ԭ�ӵĸ����ȣ���״���µ����������̼��������������ʵ�����ȣ�
��4������SO2����������������Һ��Ӧ�����������ƺ�ˮ��
��5��CO2��Na2O2��Ӧ����̼���ƺ�������
��6��NH3��������Ӧ����NO��H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2��
��7������ͭ�����ᷴӦ�����ӷ���ʽ�����ܽ�ͭ�������������Ӧ���ɵ�����������
��1����״���෨��һ�ֺ�����ķ��෨������һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״���෨��
��2����������������ȫ��Ϊ���������ӣ���NaOH��Ba(OH)2 ���ε������������Ϊ�������ӣ�������Ϊ������ӣ���BaSO4��
��3��������������̼��ˮ��Һ���磬�����������ᡢ̼���ܵ���������ƶ������ӣ���������������̼�������ܵ��룬���Զ�����������̼���ǵ���ʣ���ͬ�����Ķ�����̼����������������ԭ�ӵĸ�����Ϊ![]() ��2��
��2��![]() ��2=16:11����״���µ����������̼��������������ʵ�����ȣ������ȵ���Ħ�������ı�=44:64=11:16��
��2=16:11����״���µ����������̼��������������ʵ�����ȣ������ȵ���Ħ�������ı�=44:64=11:16��
��4������SO2����������������Һ��Ӧ�����������ƺ�ˮ����Ӧ���ӷ���ʽΪSO2+2OH-=SO32-+H2O��
��5��CO2��Na2O2��Ӧ����̼���ƺ���������Ӧ��ѧ����ʽΪ2CO2+2Na2O2= 2Na 2CO3+O2����
��6��NH3��������Ӧ����NO��H2O����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2![]() 4NO+6H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2�� 16g ���������ʵ�����0.5mol��ȫ������ԭ��ת�Ƶ���2mol��
4NO+6H2O����Ӧ����Ԫ�ػ��ϼ���0��Ϊ-2�� 16g ���������ʵ�����0.5mol��ȫ������ԭ��ת�Ƶ���2mol��
��7����Һ�������ӵ����ʵ���Ϊ0.1L��(4.0mol��L��1+1.0mol��L��1)=0.5mol��NO3-�����ʵ���Ϊ0.1L��1.0mol��L��1=0.1mol������ͭ�����ᷴӦ�����ӷ���ʽ3Cu+8H++2NO3-=3Cu2++2NO+4H2O����������Ӳ��㣬������������Ӽ����ܽ�ͭ�����ʵ���Ϊ0.15mol������Ϊ0.15mol��64g/mol= 9.6g��ͬʱ����NO����0.1mol���ڱ�״���µ����Ϊ0.1mol ��22.4L/mol=2.24L��

����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ�������У�Ԥ���������ʵ���������
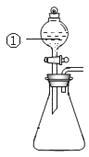
ѡ�� | �������� | �������� | Ԥ�����е����� |
A�� | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B�� | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C�� | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D�� | ������Һ | �������������Һ | ��Һ����ɫ |