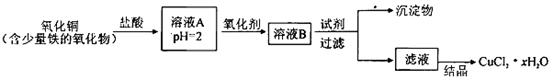
��Ŀ����
ij�о���ѧϰС��������롰�о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ������á�ʵ��̽��������ͬ����������⣺

��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣
��1��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����____________________________________��
��3��װ��E�е�������________________________________________________��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������_______��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ��Ϊ_______������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ_______��
��һ����Fe3O4 ��һ����Fe
��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����_______�����������ƣ�����_______������֤����Һ�д���Fe2+��
��̽����������������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������

��7���Լ�b�Ļ�ѧʽ��_______��
��8�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ_______��

��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣
��1��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ����____________________________________��
��3��װ��E�е�������________________________________________________��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ�ʲ�����B��ȴ��ȡ�������еĹ�����������_______��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ��Ϊ_______������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�Ϊ_______��
��һ����Fe3O4 ��һ����Fe
��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����_______�����������ƣ�����_______������֤����Һ�д���Fe2+��
��̽����������������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������

��7���Լ�b�Ļ�ѧʽ��_______��
��8�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ_______��
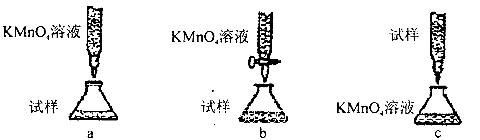
��1��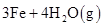
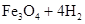
��2����ֹ����
��3����ɫ�����죬�Ҷ˹ܱ���ˮ��
��4��ϡ���� ��5���� ��
��6����ͷ�ι� ����KMnO4��Һ����Һ��ɫ
��7��NaOH ��8��77.8%
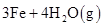
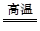
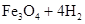
��2����ֹ����
��3����ɫ�����죬�Ҷ˹ܱ���ˮ��
��4��ϡ���� ��5���� ��
��6����ͷ�ι� ����KMnO4��Һ����Һ��ɫ
��7��NaOH ��8��77.8%
��̽��һ����1��Fe��ˮ��Ӧ�Ļ�ѧ����ʽΪ��
3Fe+4H2O��g��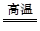 Fe3O4+4H2��
Fe3O4+4H2��
��2�����Ƭ�������Ƿ�ֹ���С�
��3��װ��E�з�����Ӧ��H2+CuO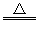 Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣
Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣
��̽����������֤��Ӧ���ɫ����ijɷ�ʱ������Fe3+������Լ�ΪKSCN��Һ����ȷ������Fe3+ʱ������Fe2+����������KMnO4��Һ����������KMnO4��Һ�����ᷢ����Ӧ���������ܽⷴӦ��ĺ�ɫ����ʱ�����������ᣬҲ���������ᣨ��ΪHNO3������Fe2+��������ϡ���ᡣ
��̽�������ɡ�����ɫ���塱֪��������ΪFe2O3����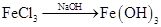 ��NaCl������
��NaCl������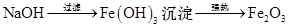 �������Լ�bΪNaOH��Һ��m��Fe2O3��="32" g,��n��Fe2O3��="0.2" mol,��n��Fe��="0.4" mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ��
�������Լ�bΪNaOH��Һ��m��Fe2O3��="32" g,��n��Fe2O3��="0.2" mol,��n��Fe��="0.4" mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ��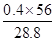 ��100%��77.8%��
��100%��77.8%��
3Fe+4H2O��g��
 Fe3O4+4H2��
Fe3O4+4H2����2�����Ƭ�������Ƿ�ֹ���С�
��3��װ��E�з�����Ӧ��H2+CuO
 Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣
Cu+H2O�������ǣ���ɫ�����죬�Ҷ˹ܱ���ˮ�顣��̽����������֤��Ӧ���ɫ����ijɷ�ʱ������Fe3+������Լ�ΪKSCN��Һ����ȷ������Fe3+ʱ������Fe2+����������KMnO4��Һ����������KMnO4��Һ�����ᷢ����Ӧ���������ܽⷴӦ��ĺ�ɫ����ʱ�����������ᣬҲ���������ᣨ��ΪHNO3������Fe2+��������ϡ���ᡣ
��̽�������ɡ�����ɫ���塱֪��������ΪFe2O3����
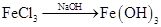 ��NaCl������
��NaCl������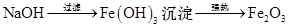 �������Լ�bΪNaOH��Һ��m��Fe2O3��="32" g,��n��Fe2O3��="0.2" mol,��n��Fe��="0.4" mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ��
�������Լ�bΪNaOH��Һ��m��Fe2O3��="32" g,��n��Fe2O3��="0.2" mol,��n��Fe��="0.4" mol,��Ӧ��Bװ������Ԫ�ص���������Ϊ��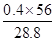 ��100%��77.8%��
��100%��77.8%��
��ϰ��ϵ�д�
�����Ŀ
 3Zn��2K2FeO4��8H2O���õ�طŵ�ʱ�ĸ�����ӦʽΪ________�����ʱ����������Һ��pH________�����������䡱��С������
3Zn��2K2FeO4��8H2O���õ�طŵ�ʱ�ĸ�����ӦʽΪ________�����ʱ����������Һ��pH________�����������䡱��С������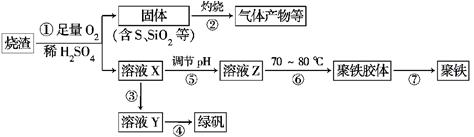




 xH2O)�������²�����
xH2O)�������²�����