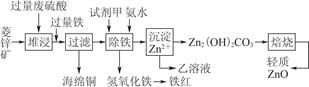
��Ŀ����
���������г��������᳧���ջ�����ʯ������(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)���Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)�������� �£�
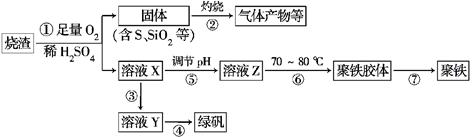
(1)�����̢��в���������ͨ��������Һ�У���Һ����ɫ����________(��ѡ�����)��
a��Ʒ����Һ b����ɫʯ����Һ c������KMnO4��Һ d����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ______________________
(3)���̢��У�������������____________����Ӧ�Ļ�ѧ����ʽΪ_____________________
����ҺY���̷�ʱ����ȡ����Y��Һ���Թ��У����Թ��ڼ���������________________��Һ���۲���Һ�Ƿ��Ϊ________ɫ������֤�����Ƿ���Fe3����
(4)��ʵ�������ɹ��̢��е�____________(���������)����Ҫʹ�þƾ��ơ����żܡ�����ǯ�ȣ�����Ҫ�IJ���������___________________________��
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ����____________________________________________________��
(6)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�飺���÷�����ƽ��ȡ2.700 g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)SO4]n���������Ʒ����Ԫ�ص���������Ϊ________��(���������в�����Ԫ�غ���Ԫ��)

(1)�����̢��в���������ͨ��������Һ�У���Һ����ɫ����________(��ѡ�����)��
a��Ʒ����Һ b����ɫʯ����Һ c������KMnO4��Һ d����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ______________________
(3)���̢��У�������������____________����Ӧ�Ļ�ѧ����ʽΪ_____________________
����ҺY���̷�ʱ����ȡ����Y��Һ���Թ��У����Թ��ڼ���������________________��Һ���۲���Һ�Ƿ��Ϊ________ɫ������֤�����Ƿ���Fe3����
(4)��ʵ�������ɹ��̢��е�____________(���������)����Ҫʹ�þƾ��ơ����żܡ�����ǯ�ȣ�����Ҫ�IJ���������___________________________��
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ����____________________________________________________��
(6)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�飺���÷�����ƽ��ȡ2.700 g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)SO4]n���������Ʒ����Ԫ�ص���������Ϊ________��(���������в�����Ԫ�غ���Ԫ��)
(1)acd��(2)4FeS��3O2��12H��=4Fe3����6H2O��4S��(3)Fe(����)��Fe2(SO4)3��Fe=3FeSO4
���軯��(������������)���졡(4)�����ᾧ��������������(5)�ٽ�Fe3����ˮ�⡡(6)31.1 %
���軯��(������������)���졡(4)�����ᾧ��������������(5)�ٽ�Fe3����ˮ�⡡(6)31.1 %
��������Ϣ��֪�����̢��������м�ϡ���Ტͬʱͨ����������������������������ܽ⣬�������������������ˮ��������ҺX����������Һ�����̢����չ��壬���е��������ɶ�������(1)���������ֱܷ�ʹƷ����Һ������KMnO4��Һ����ˮ��ɫ��(2)���̢��У�FeS��O2��H2SO4��Ӧ�����ӷ���ʽΪ4FeS��3O2��12H��=4Fe3����6H2O��4S��(3)�ɲ����̷����ƿ�֪���̢��ǽ�������ת��Ϊ���������Ĺ��̣�Ӧ�������ۻ�ԭ���������йط�Ӧ�Ļ�ѧ����ʽΪFe2(SO4)3��Fe=3FeSO4����֤����������Һ���Ƿ���Fe3�������ú�SCN������Һ���飬����Һ��Ϊ��ɫ��֤����Һ�к���Fe3��������Һ����ɫ��֤����Һ�в���Fe3����(4)������������Һ���̷���Ӧʹ�������ᾧ�ķ�������Ҫʹ�þƾ��ơ����żܡ������������ȡ�(5)���̢��У�����������Һ���ȵ�70��80�棬Ŀ���Ǵٽ�Fe3����ˮ�⡣(6)3.495 g��BaSO4Ϊ0.015 mol����Ԫ�ص�����Ϊ56 g��mol��1��0.015 mol��0.840 g�����Ըþ�����Ʒ����Ԫ�ص���������Ϊ��0.840/2.700����100%��31.1 %��

��ϰ��ϵ�д�
�����Ŀ
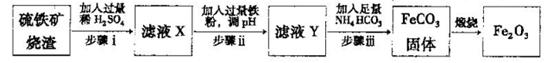
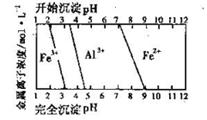

 FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
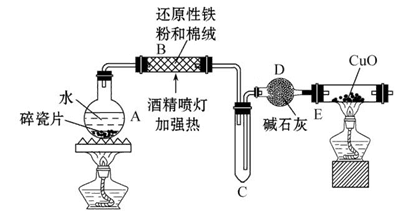
FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______�� Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����:
Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����: