��Ŀ����
16����20mL����������Һ����μ���0.2mol/L������Һ���ζ�������ͼ��ʾ����˵������ȷ���ǣ�������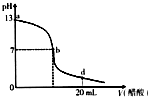
| A�� | ������������Һ�����ʵ���Ũ��Ϊ0.1 mol/L | |
| B�� | ��b�㣬c��Na+��=c��CH3COO-�� | |
| C�� | ��d�㣬��Һ����������Ũ���ɴ�С��˳��Ϊc��CH3COO-����c��Na+����c��H+����c��OH-�� | |
| D�� | ����������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ������b��d���ij�� |
���� A���ɼ���Һ��pH��������������ҺŨ�ȣ�
B��b��ʱ��Һ�����ԣ����ݵ���غ������
C��d��ʱ��������Һ������Լ�����غ������
D�����������ƺʹ���ǡ�÷�Ӧʱ���ߵ����ʵ�����ȣ�
��� �⣺A����δ�Ӵ���ʱ������������Һ��pH=13��������������Һ������������Ũ����0.1mol/L������������ǿ����ʣ���������������ҺŨ����0.1mol/L����A��ȷ��
B��b��ʱ��Һ�����ԣ���c ��H+��=c ��OH-�������ݵ���غ��c ��Na+��=c��CH3COO-������B��ȷ��
C��d��ʱ����Һ�����ԣ���c ��H+����c ��OH-�������ݵ���غ��c ��CH3COO����c ��Na+�����õ���Һ�е������Ǵ����ƺʹ��ᣬ�����ʵ�����ȣ�������������ʣ���������ǿ����ʣ�����c ��Na+����c ��H+����������Ũ�ȴ�С˳����c ��CH3COO����c ��Na+����c ��H+����c ��OH-������C��ȷ��
D�����������ƺʹ���ǡ�÷�Ӧʱ���ߵ����ʵ�����ȣ���������ʵ���Ũ����0.2mol/L������������Һ��Ũ����0.1mol/L��Ҫʹ���ߵ����ʵ�����ȣ����������Ƶ����Ӧ���Ǵ����2��������Ϊ10mL������Һ������ʱ��������������������������Һ�������Һǡ����ȫ��Ӧ�ĵ�λ��0-b֮�䣬��D����
��ѡ��D��
���� ���⿼�����������Һ�����жϣ�����������ʵĵ����ص㡢��Һ�е����ʼ�����غ���������ɣ��Ѷ��еȣ�

| A�� | ��λʱ��������nmolA2��ͬʱ����2n molAB | |
| B�� | ������ѹǿ����ʱ����仯 | |
| C�� | AB���������ʵ���A2���������� | |
| D�� | �����и���ֵ������������ʱ��仯 |
| A�� | 2��4 | B�� | 3��4 | C�� | 2��3 | D�� | ��ȷ�� |
| A�� | ����Ԫ����ɿ��ж��ǻ����� | |
| B�� | ��������������������ַ����ε���ɣ��ʿ��������� | |
| C�� | �����������������������ַ����ε���ɣ��ʿ����������� | |
| D�� | NaHSO4����ˮ�ɵ���������ӹʿ��Գ�Ϊ�� |
| A�� | ±���е��ʴ�������Ȼ���� | |
| B�� | ��˵�������ӣ������۷е����� | |
| C�� | ��˵�������ӣ�������������ǿ | |
| D�� | ������ˮ��Ӧ��������ͨʽX2+H2O�THX+HXO��ʾ |
| �¶�/�� | 25 | t1 | t2 |
| Kw/mol2•L-2 | 1��10-14 | a | 1��10-12 |
��1����25��t1��t2����a��1��10-14�����������������=������
��2����25���£�0.05mol/L��Ba��OH��2��Һ��pH=13��
��3����25���£�pH=10��NaOH��Һ�У�ˮ���������c��OH-��Ϊ��1��10-10mol/L��
��4����t2���£���pH=9������������ҺV1L��pH=4��������ҺV2L��ϣ����Ϻ���Һ���Ϊԭ����Һ���֮�ͣ�������Һ��pH=7����V1��V2=$\frac{1}{9}$��
| A�� | ����������䣬����H2O��g�������ʵ��� | |
| B�� | �������������Сһ�� | |
| C�� | ����������䣬����N2ʹ��ϵѹǿ���� | |
| D�� | ����ѹǿ���䣬����N2ʹ���������� |
| A�� | ����ѹǿ����ѧƽ�ⲻһ�������ƶ� | |
| B�� | ͨ�뺤������ѧƽ�ⲻһ�������ƶ� | |
| C�� | ����Y�����ʵ�������ѧƽ��һ�������ƶ� | |
| D�� | �����������䣬�����¶ȣ���ѧƽ��һ�������ƶ� |
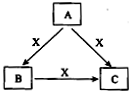 ��A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ�� ��A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ�
��A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ�� ��A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ�