��Ŀ����
��8�֣�A��B��C����Ԫ�ص�ԭ�Ӿ�����ͬ�ĵ��Ӳ�������B�ĺ˵������A��1��Cԭ�ӵĵ���������Bԭ�ӵĵ���������4�� 1molA�ĵ��ʸ��������ᷴӦ�����û�����״����22.4L��H2����ʱAת��Ϊ����ԭ�Ӿ�����ͬ���Ӳ�ṹ�����ӡ���ش�
��1���õ���ʽ��ʾA��C��ɵĻ�������γɹ���Ϊ______________________��
��2��B���ӵĵ���ʽ�� ����B�����Ӿ�����ͬ�������ķ����У���һ�ַ��ӿ������ữ�������Σ��÷��ӵĵ���ʽ�� ��
��3�����ڱ�����C�������ڵ�ͬ��Ԫ���γɵ���̬�⻯���У��е���ߵ��� �������⻯�ﻯѧʽ����ԭ���� ��
��4��д��B����������ˮ�����C����������ˮ����֮�䷴Ӧ�����ӷ���ʽ�������漰�ĺ�CԪ�ص����ʾ�������ˮ���� ��
(1) 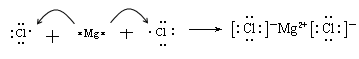
(2) Al3+ 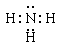
(3) HF HF����֮�������� ��4��Al(OH)3+3H+=Al3++3H2O
����

��ϰ��ϵ�д�
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ

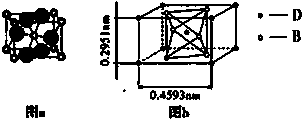
 ��ͼ�����ڱ��ж����ڵ�һ���֣�A��B��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������2��������Ԫ�ط��ű�ʾ��
��ͼ�����ڱ��ж����ڵ�һ���֣�A��B��C����Ԫ�ص�ԭ�Ӻ��������֮�͵���B����������2��������Ԫ�ط��ű�ʾ��