��Ŀ����
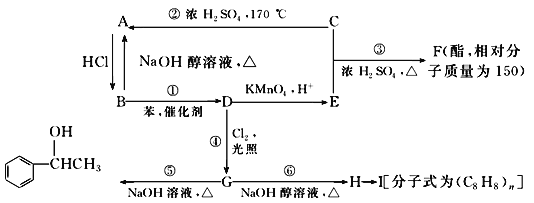
����Ŀ����֪������±�����ڴ����������¿��������������±���⣬C�������г������л��75%��C��Һ������ҽ���������ұ��ܱ����Ը��������Һ����Ϊ�����ᣮ��������֮���ת����ϵ��ͼ��ʾ�����������������������ȥ����
��ش��������⣺
��1��д�����ʵĽṹ��ʽ��D________��I________��
��2���ڢ١���6����Ӧ�У�������ȥ��Ӧ����________�����ţ�
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��______________________________________��
��______________________________________��
��4��д��һ�ַ�������Ҫ���F��ͬ���칹��Ľṹ��ʽ��F��ͬ���칹�����������࣬�ܷ���������Ӧ���ұ����ϵ�һ��ȡ����ֻ�����֣�______________________________��
���𰸡�![]()
 �ڢ�
�ڢ� ![]()
![]() +NaOH
+NaOH![]()
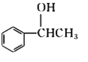 +NaCl
+NaCl 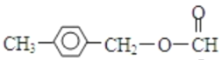 ��
��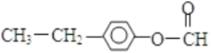 ��
��
��������
C�������г������л��75%��C��Һ������ҽ������, CΪCH3CH2OH,��Ϸ�Ӧ����֪AΪCH2=CH2����A��HCl�����ӳɷ�Ӧ����B��B�ܷ�����ȥ��Ӧ����A����BΪCH3CH2Cl��B������Ӧ����D��D����һϵ�з�Ӧ����I��H�����Ӿ۷�Ӧ����H����D��H��̼ԭ�Ӹ�������8��B����ȡ����Ӧ����D����DΪ![]() ��D����������Ӧ����EΪ
��D����������Ӧ����EΪ![]() ��C��E����������Ӧ����F������F��Է�������֪��F�ṹ��ʽΪ
��C��E����������Ӧ����F������F��Է�������֪��F�ṹ��ʽΪ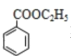 ��D����ȡ����Ӧ����G��G����ȡ����Ӧ����
��D����ȡ����Ӧ����G��G����ȡ����Ӧ����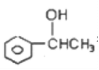 ����GΪ
����GΪ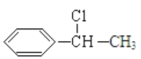 ��G������ȥ��Ӧ����HΪ
��G������ȥ��Ӧ����HΪ![]() ��H�����Ӿ۷�Ӧ���ɸ߾���IΪ
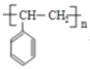
��H�����Ӿ۷�Ӧ���ɸ߾���IΪ ���ݴ˷������
���ݴ˷������
��1��ͨ�����Ϸ���֪D![]() ��I
��I 
���ʴ�Ϊ��![]() ��
�� ��
��
��2���ڢ١���6����Ӧ�У�������ȥ��Ӧ���Ǣڢޣ��ʴ�Ϊ���ڢޣ�
��3����Ϊ������ͱ���ȡ����Ӧ����Ӧ����ʽΪ�� ��
��
�� ��Ӧ����ʽΪ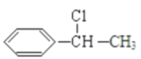 +NaOH/span>
+NaOH/span>![]()
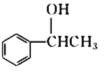 +NaCl���ʴ�Ϊ��
+NaCl���ʴ�Ϊ��![]() ��
��![]() +NaOH
+NaOH![]()
 +NaCl
+NaCl
��4�� F��ͬ���칹������������,�ܷ���������Ӧ,�ұ����ϵ�һ��ȡ����ֻ������,˵��������Ϊ������,���ܵĽṹΪ��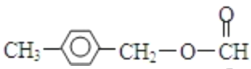 ��
��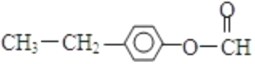 ��
��
�ʴ�Ϊ��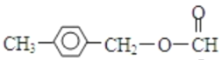 ��
�� ��
��