��Ŀ����
2����һƿ�������Һ�����п��ܺ���NH4+��Na+��Ba2+��Al3+��Fe3+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺��1��ȡpH��ֽ���飬��Һ��ǿ���ԣ������ų�CO32-��AlO2-���ӵĴ��ڣ�
��2��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ�������ų�Fe3+��NO3-���ӵĴ��ڣ�
��3��д����2���������ķ�Ӧ�����ӷ���ʽ2I-+Cl2=I2+2Cl-��
��4����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ���������������ֿ����ų�Al3+���ӵĴ��ڣ�
��5��ȡ����4����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ����ų�SO42-���ӵĴ��ڣ�
��6������4���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ��������Һ�п϶����ڵ�������NH4+��Ba2+��I-��
���� ��1����pH��ֽ���飬������Һ����ǿ���ԣ�CO32-��AlO2-�ܹ��������ӷ�Ӧ������Һ�в�����ڣ�
��2�����Ȼ�̼��Һ���Ϻ�ɫ��˵��������ˮ���еⵥ�����ɣ�ԭ��Һ��һ������I-��������������ܽ������������������棬����һ������Fe3+��NO3-��
��3�������������������ɵⵥ�ʣ�
��4����������������Һ�Ĺ�����û�г������ɣ�˵��һ��������Al3+��
��5�����Ba2+����̼���Ʒ�Ӧ�����������жϴ��ڵ����ӣ��������ӹ����жϲ��ܴ��ڵ����ӣ�
��6����ʹʪ��ĺ�ɫʯ����ֽ�����������ǰ���������Ӧ���ɰ�����������笠����ݴ˽�ɣ�
��� �⣺��1����pH��ֽ���飬������Һ����ǿ���ԣ�CO32-��AlO2-�ܹ��������ӷ�Ӧ������Һ�в�����ڣ��ʴ�Ϊ��CO32-��AlO2-��
��2�����Ȼ�̼��Һ���Ϻ�ɫ��˵��������ˮ���еⵥ�����ɣ�ԭ��Һ��һ������I-��������������ܽ������������������棬����һ������Fe3+��NO3-���ʴ�Ϊ��NO3-��Fe3+��
��3������������ӷ�Ӧ���ɵⵥ�ʣ����ӷ�ӦΪ2I-+Cl2=I2+2Cl-���ʴ�Ϊ��2I-+Cl2=I2+2Cl-��
��4����������������Һ�Ĺ�����û�г������ɣ�˵��һ��������Al3+���ʴ�Ϊ��Al3+��
��5�����֣�4���еļ�����Һ����Na2CO3��Һ���а�ɫ�������ɣ�Ba2+����̼���Ʒ�Ӧ�����������жϴ��ڵ����ӣ����Դ��ڱ����ӣ�һ����������������ӣ�
�ʴ�Ϊ��SO42-��
��6��������ʹ��ɫʯ����ֽ����������Һ��һ������笠����ۺ����Ϸ�����һ�����ڵ������ǣ�NH4+��Ba2+��I-���ʴ�Ϊ��NH4+��Ba2+��I-��
���� ������Ҫ�����˳������ӵļ��鷽������Ŀ�Ѷ��еȣ�ע�����ճ������ӵĻ�ѧ���ʼ����鷽�����ܹ��������ӹ��桢���ӷ�Ӧ�����ж����ӹ����������ȷ��������ʱ�������ų��������ӣ�ȷ�����鷽���������ԣ�

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�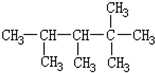
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
| A�� | ԭ��������24 | |
| B�� | �����������SeO3�������������� | |
| C�� | ԭ�Ӱ뾶�ȸ�С | |
| D�� | ��̬�⻯�ﻯѧʽ��H2Se���ȶ��Ա�HCl�� |
| A�� | �����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��Na2CO3��Һ��NaHCO3��Һ��������������Һ�У�2c��OH-��-2c��H+��=3c��H2CO3��+c��${HCO}_{3}^{-}$��-c��${CO}_{3}^{2-}$�� | |
| B�� | pH=2��HA����Һ��pH=12��MOH����Һ�������ϣ�c��M+��=c��OH-����c��H+��=c��A-�� | |
| C�� | ��Ũ�ȡ��������Na2CO3��NaHCO3��ϣ�$\frac{c��H{CO}_{3}^{-}��}{c��{H}_{2}C{O}_{3}��}$��$\frac{c{��CO}_{3}^{2-}��}{c{��HCO}_{3}^{-}��}$ | |
| D�� | ������AgCl�ֱ���룺��5mLˮ��10mL0.2mol/LMgCl2��20mL0.3mol/L�������ܽ������ͣ�c��Ag+�����٣��ڣ��� |
| A�� | һ�������£�Cl2���ڼױ��ı���������Ϸ���ȡ����Ӧ | |
| B�� | ��������Һ��ͨ������CO2���������ɱ��Ӻ�̼���� | |
| C�� | ����ͱ�ϩ�����ʵ�����1 mol����ȫȼ������3 mol H2O | |
| D�� | ������2��2������������Br2��Ӧ����һ��ȡ����ֻ��һ�� |
 ��DCH4��ECH3CH3��
��DCH4��ECH3CH3��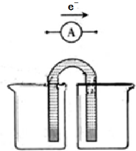 ��1�����������һ�����Ϳɳ���أ�����ͨ�����ȣ��õ���ܽϳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O$?_{���}^{�ŵ�}$3Zn��OH��2+2Fe��OH��3+4KOH��������صĸ���������п���ŵ�ʱ������������ԭ�����������ԭ������Ӧ��
��1�����������һ�����Ϳɳ���أ�����ͨ�����ȣ��õ���ܽϳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O$?_{���}^{�ŵ�}$3Zn��OH��2+2Fe��OH��3+4KOH��������صĸ���������п���ŵ�ʱ������������ԭ�����������ԭ������Ӧ��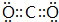 ���õ���ʽ��ʾ������ C2D ���γɹ���
���õ���ʽ��ʾ������ C2D ���γɹ��� ��
��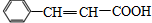
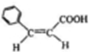 ��
��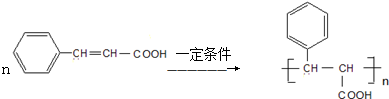 ��
��