��Ŀ����
��ͼΪ��ҵ�Ʊ�������豸ʾ��ͼ�������������з����ķ�Ӧ��Ҫ�У�
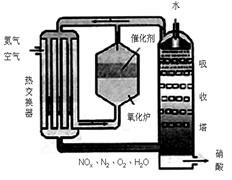
��4NH3��g��+5O2��g�� 4NO��g��+6H2O��l�� ��H��0
4NO��g��+6H2O��l�� ��H��0
��2NO��g��+O2��g�� 2NO2��g�� ��H��0
2NO2��g�� ��H��0
��3NO2��g��+H2O��l�� 2HNO3��l�� +NO��g�� ��H��0
2HNO3��l�� +NO��g�� ��H��0
��1����ʹ�������ڷ�Ӧ���������������HNO3���ʵĴ�ʩ�� ��
��2����2L�ܱ������ڳ���0��50 mol NO��0��25 mol O2��ά�ַ�Ӧ�¶�Ϊ800�棬����Ӧ�ﵽƽ��ʱ��NO��ת����Ϊ50������800��ʱ��Ӧ2NO��O2��2NO2��ƽ�ⳣ��K�� ��
��3��ij����ÿ���豸ÿСʱ������20 t 63�������ᣨ�ܶ�Ϊ1��4 g/cm3�������蹤ҵ������������У�ͨ��ѭ����������ʹNH3��O2������ȫ���á�
�ش��������⣺
�ٸù����豸��������������ʵ���Ũ���� ��
��ÿСʱ�����������µ�ˮ������Ӧ�Ƕ��ٶ֣�

��4NH3��g��+5O2��g��
 4NO��g��+6H2O��l�� ��H��0
4NO��g��+6H2O��l�� ��H��0��2NO��g��+O2��g��
 2NO2��g�� ��H��0
2NO2��g�� ��H��0��3NO2��g��+H2O��l��
 2HNO3��l�� +NO��g�� ��H��0
2HNO3��l�� +NO��g�� ��H��0��1����ʹ�������ڷ�Ӧ���������������HNO3���ʵĴ�ʩ�� ��
| A���ʵ������¶� | B���ʵ������������ڵ�ѹǿ |
| C�����������O2��Ũ�� | D�������������ɻ���������Һ�Ӵ��� |
��3��ij����ÿ���豸ÿСʱ������20 t 63�������ᣨ�ܶ�Ϊ1��4 g/cm3�������蹤ҵ������������У�ͨ��ѭ����������ʹNH3��O2������ȫ���á�
�ش��������⣺
�ٸù����豸��������������ʵ���Ũ���� ��
��ÿСʱ�����������µ�ˮ������Ӧ�Ƕ��ٶ֣�
��1��BC ��2��16 ��ÿ��2�֣���4�֣�
��3����14 mol/L ��4�֣�
��ÿСʱ���ɵ����������m��HNO3��=" 20" t��63��=12��6 t
�����ܻ�ѧ����ʽ��NH3+2O2=HNO3+H2O
ÿСʱ����ˮm��H2O��1= 12��6 t��18/63=3��6 t
���ɵ�����������ˮ������m��H2O��=" 20" t��12��6 t=7��4 t
ÿСʱ�����������µ�ˮ������m��H2O��2= 7��4 t ��3��6 t=3��8 t ��4�֣�
��3����14 mol/L ��4�֣�
��ÿСʱ���ɵ����������m��HNO3��=" 20" t��63��=12��6 t
�����ܻ�ѧ����ʽ��NH3+2O2=HNO3+H2O
ÿСʱ����ˮm��H2O��1= 12��6 t��18/63=3��6 t
���ɵ�����������ˮ������m��H2O��=" 20" t��12��6 t=7��4 t
ÿСʱ�����������µ�ˮ������m��H2O��2= 7��4 t ��3��6 t=3��8 t ��4�֣�
��

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ
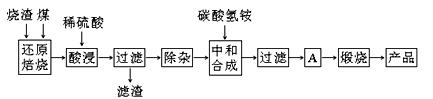
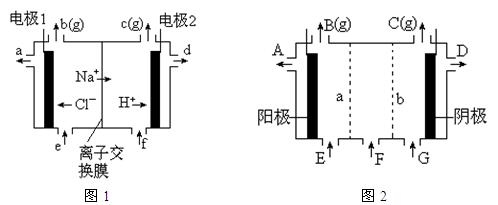
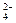 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
CH3CH2OH(g)+H2O(g)����һ��ѹǿ�£��÷�Ӧ��һЩʵ���������±���
 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��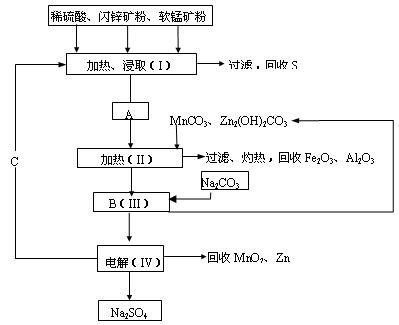
 2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4" kJ/mol �ݴ˻ش��������⣺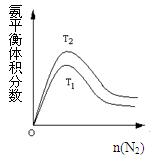
 2(g)+CO(g) ��ƽ�ⳣ��K1
2(g)+CO(g) ��ƽ�ⳣ��K1