��Ŀ����
������������������������������в����Ĺ�ҵ��������Ҫ��Fe2O3������SiO2��Al2O3��CaO��MgO�����ʣ����ø�������ȡҩ�ø��ϡ������������Ĺ����������£�
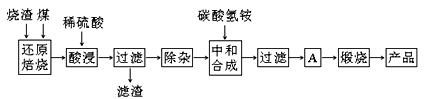
��1���ڡ���ԭ���ա��в������ж���������� ��
��2���������ʱ��һ�㲻����20 min�����ڿ��������ʱ���������Һ��Fe2+�������½�����ԭ�������ӷ���ʽ��ʾ�� ��
��3�������±����ݣ�
�ڡ����ӡ������У�Ϊ��ȥFe3+��Al3+����Һ��pH���ֵӦС�� ����pH��5ʱ����Һ��c(Al3+)Ϊ mol��L��1����֪������Ksp[Al(OH)3]��2.0��10��33����
��4�����кͺϳɡ���Ŀ���ǽ���Һ��Fe2+ת��Ϊ̼������������̼�����������������Ӧ�����ӷ���ʽΪ ��
���õ����ʵ�����̼������̼����泥����Ʒ�п��ܻ��е������� ��
��5��A�IJ����� ��
��6��m g���������������տɵú�������n g��ҩ����涨���Ƶõĺ��������к���������������98.0%������ѡ�õ�������������������Ӧ������ ���ú�m��n�ı���ʽ��ʾ����

��1���ڡ���ԭ���ա��в������ж���������� ��
��2���������ʱ��һ�㲻����20 min�����ڿ��������ʱ���������Һ��Fe2+�������½�����ԭ�������ӷ���ʽ��ʾ�� ��
��3�������±����ݣ�
| �������� | Al(OH)3 | Mg(OH)2 | Fe(OH)3 | Fe(OH)2 |
| ��ʼ������pH | 3.10 | 8.54 | 2.01 | 7.11 |
| ��ȫ������pH | 4.77 | 11.04 | 3.68 | 9.61 |
��4�����кͺϳɡ���Ŀ���ǽ���Һ��Fe2+ת��Ϊ̼������������̼�����������������Ӧ�����ӷ���ʽΪ ��
���õ����ʵ�����̼������̼����泥����Ʒ�п��ܻ��е������� ��
��5��A�IJ����� ��
��6��m g���������������տɵú�������n g��ҩ����涨���Ƶõĺ��������к���������������98.0%������ѡ�õ�������������������Ӧ������ ���ú�m��n�ı���ʽ��ʾ����
��1��CO��SO2�ȣ�д��һ��������Ҳ���֣� ��1�֣�
��2��4Fe2++O2+4H+��4Fe3++2H2O ��3�֣�
��3��7.11 ��2�֣�
2.0��10��6 ��2�֣�
��4��Fe2++2HCO3����FeCO3�� +CO2��+H2O ��3�֣�
+CO2��+H2O ��3�֣�
CaO MgO ��2�֣�
��5��ϴ�ӡ����ֻдϴ��Ҳ���֣� ��1�֣�
��6��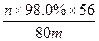 �����������𰸾����֣� ��2�֣�
�����������𰸾����֣� ��2�֣�
��2��4Fe2++O2+4H+��4Fe3++2H2O ��3�֣�
��3��7.11 ��2�֣�
2.0��10��6 ��2�֣�
��4��Fe2++2HCO3����FeCO3��
 +CO2��+H2O ��3�֣�
+CO2��+H2O ��3�֣�CaO MgO ��2�֣�
��5��ϴ�ӡ����ֻдϴ��Ҳ���֣� ��1�֣�
��6��
 �����������𰸾����֣� ��2�֣�
�����������𰸾����֣� ��2�֣���

��ϰ��ϵ�д�
�����Ŀ


 ���ͣ������ա�
���ͣ������ա�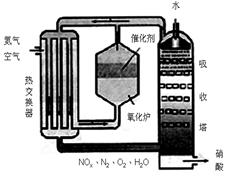
 4NO��g��+6H2O��l�� ��H��0
4NO��g��+6H2O��l�� ��H��0
 -1
-1 �أ��õ�ZnO 2.43g�ͱ�״����CO20.224l����ʽ̼��п�Ļ�ѧʽ
�أ��õ�ZnO 2.43g�ͱ�״����CO20.224l����ʽ̼��п�Ļ�ѧʽ