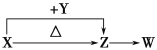��Ŀ����
����Ŀ��ClO2��NaClO2������Ư��������ҵ����ClO2������ȡNaClO2����Ĺ���������ͼ��ʾ������˵���������

A. ͨ��Ŀ����ɽ��������в�����ClO2ȫ�����ϵ���������
B. ������������NaClO2�����ӷ���ʽΪ2ClO2+H2O2=2ClO2-+2H++O2��
C. ����a�IJ����������ˡ�ϴ�Ӻ���
D. ��ҵ�Ͻ�ClO2�����Ƴ�NaClO2����������ҪĿ���DZ������������
���𰸡�B
����������ClO2��������һ������ClO2���壬ͨ����������Խ��䴵���������н������գ�ѡ��A��ȷ���������м�����Ũ����������Һ����Ȼ���еķ�Ӧ�����ܵõ������ӣ�ѡ��B������ȴ�ᾧ�õ�NaClO2�����Ӧ�þ������ˣ�ϴ�ӣ�����õ���Ʒ��ѡ��C��ȷ���������������䶼Զ�ȹ������ѣ����Խ�ClO2�����Ƴ�NaClO2�������ҪĿ���DZ�����������䣬ѡ��D��ȷ��

��ϰ��ϵ�д�
�����Ŀ