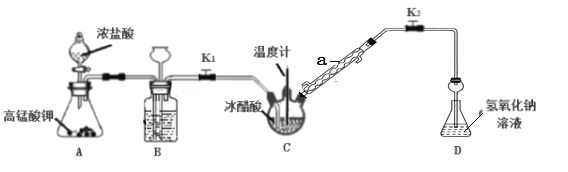
��Ŀ����
����Ŀ����֪һ��������п����1.0Lϡ���ᷴӦ(���ȷ�Ӧ)������H2�����ʵ����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ�����н��۲���ȷ����

A. ����п����Ϊп�ۣ�.���Լӿ����H2�ķ�Ӧ����
B. ��Ӧǰ4 min���¶ȶԷ�Ӧ���ʵ�Ӱ���Ũ�ȴ�
C. ��Ӧǰ4 min��ƽ������v(HC1)=0.18 mol��L-1��min-1
D. ��÷�Ӧ�м��������CuSO4���壬��ʹ�������������ʼӿ�
���𰸡�D
��������A������п���ij�п�ۣ������˷�Ӧ��ĽӴ���������Լӿ����H2�����ʣ���A��ȷ��B���÷�ӦΪ���ȷ�Ӧ�����ǰ4min���¶ȶԷ�Ӧ���ʵ�Ӱ��Ƚϴ�B��ȷ��C����Ӧǰ4min������H2�����ʵ���Ϊ0.36mol�������ĵ�����Ϊ0.72mol����Ӧǰ4 min��ƽ������v(HC1)=![]() =0.18 mol��L-1��min-1����C��ȷ��D����÷�Ӧ�м��������CuSO4���壬п���û���ͭ������п���ı��棬��ֹп������Ľ�һ����Ӧ����ʹ�������������ʼ�С����D����ѡD��
=0.18 mol��L-1��min-1����C��ȷ��D����÷�Ӧ�м��������CuSO4���壬п���û���ͭ������п���ı��棬��ֹп������Ľ�һ����Ӧ����ʹ�������������ʼ�С����D����ѡD��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�����Ŀ��ij��ҵ�����к��� Cr(OH)3��Al2O3��CuO��NiO �����ʣ���ҵ��ͨ���������̻����������õĽ�������ȡ Na2Cr2O7��

��֪������ˮ�������������Һ�д��� Na2CrO4��NaAlO2 ������
�ڳ�ȥ���� II ����Һ�д��ڷ�Ӧ 2CrO42-+2H+![]() Cr2O72-+H2O
Cr2O72-+H2O
��Na2Cr2O7��Na2CrO4 �ڲ�ͬ�¶��µ��ܽ��(g/100 g H2O)���±���
20�� | 60�� | 100�� | |
Na2Cr2O7 | 183 | 269 | 415 |
Na2CrO4 | 84 | 115 | 126 |
(1)������������������ NaAlO2 �Ļ�ѧ����ʽΪ_____��
(2)��������ʱ�����������ϡ���������Һ�� pH ��ȥ AlO2 -����ϡ��������������������Ӧ�����ӷ���ʽΪ_______________________��
(3)��ϵ�в�����Ϊ����������ϡ���ᡢ_____����ȴ�ᾧ�����ˡ���������ϡ�����Ŀ����_________������ III �г��������������ƾ����⣬��Ҫ�ɷ��� _________________(д��ѧʽ)��
(4)��ҵ�ϻ���������ˮ�������������Һ�м�������ϡ���ᣬ��ʯī���缫��������������������ĵ缫��ӦʽΪ ____��
(5)����ͼ��������Һ������Ȼ�в����� Na2Cr2O7��Cr Ϊ�ؽ���Ԫ�أ����������߾���������У� ��Ժ�ˮ����ؽ�����Ⱦ��Ϊ�ⶨ����Һ���е� c(Na2Cr2O7)��ijʵ��С��ȡ����Һ��20 mL����ˮϡ���� 250 mL����ȡϡ�ͺ����Һ 25 mL ����ƿ�У��� c mol/L�� FeSO4 ��Һ����������ԭ�ζ�,���յ� ʱ��� FeSO4 ��Һ���Ϊ V mL[��֪�����ķ�ӦΪNa2Cr2O7+FeSO4+H2SO4��Na2SO4+Cr2(SO4)3+ Fe2(SO4)3+ H2O(δ��ƽ)]��������Һ���е�c(Na2Cr2O7)=_________mol/L��