��Ŀ����
����Ŀ��±�ص��ʼ��仯�������������粻��ȱ�ٵ���ɲ��֡���������ѧ���ʽṹ�����ʵ����֪ʶ�ش��������⣺
��1��Atԭ�ӵĺ���۵����Ų�ʽΪ______________________��I3+����ԭ���ӻ���ʽΪ_______��ClO2-�Ŀռ乹��Ϊ__________����̬��ԭ�Ӻ��������________�ֿռ��˶�״̬��
��2����֪�ߵ��������ֽṹ����ѧʽ�ֱ�ΪH5IO6��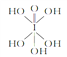 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��Ƚ϶��ߵ�����ǿ����H5IO6 ______HIO4���>������=����<����.
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��Ƚ϶��ߵ�����ǿ����H5IO6 ______HIO4���>������=����<����.
��3��TiCl4�۵�Ϊ-24�棬�е�Ϊ136.4�档�����ڼױ�����̬TiCl4����_________���塣
��4����п��ԭTiCl4��������Һ�����Ƶ���ɫ�����[TiCl(H2O)5]Cl2��H2O��1mol��������к��ЦҼ���ĿΪ__________��
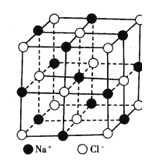
��5��CaF2�����ṹ����ͼ
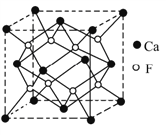
�پ����о��������2��Ca2+(X)��һ��F-(Y)�γɵļнǣ���XYX��Ϊ___________��
��CaF2��Ħ������Ϊ78g/mol�������ܶ�Ϊ��g/cm3�������ӵ�����ΪNA�����������������Ca2+�����ľ���Ϊ____________cm
���𰸡� 6s26p5 sp3 V�� 5 < ���� 18NA 109��28�� ![]()
����������1��Atԭ�ӵĺ˵����Ϊ85����������Ų�Ϊ[Xe] 6s26p5,����۵����Ų�ʽΪ6s26p5 ��I3+����ԭ��I�ļ۲���Ӷ���Ϊ4������2���µ��Ӷԣ��ӻ���ʽΪsp3 �� ClO2-������ԭ�ӵļ۲���Ӷ���Ϊ4���µ��Ӷ���Ϊ2������sp3���ռ乹��ΪV�ͣ���ԭ�Ӻ˵����Ϊ9�������Ų�Ϊ1s22s22p5�����������5�ֿռ��˶�״̬����ȷ�𰸣�6s26p5 �� sp3 �� V�� �� 5��
��2������ԭ���Ϸ��ǻ���ԭ����ĿԽ�������ǻ�������Խǿ�ң�����Խǿ����������ǿ����H5IO6 <HIO4����ȷ�𰸣�<��
��3�����ݸ�����Ϣ��֪��TiCl4���۷е�ϵͣ����ڷ��Ӿ��壻��ȷ�𰸣����Ӿ��塣
��4��1molTi��1molCl��5molH2O���γ�6mol������5molH2O�ں���5��2=10mol���������1molH2O�ں���2mol������������6+10+2=18mol����ĿΪ18NA����ȷ�𰸣�18NA��
��5����1��F��4��Ca�γɿռ���������ṹ����ϼ���ļ��ǿ�֪�þ����о��������2��Ca2+(X)��һ��F-(Y)�γɵļнǣ���XYX��Ϊ109.5����109��28������ȷ����109��28����
���ɾ��徧��ͼ��Xλ��������Ķ�������ϣ�Yλ�����������ģ���ÿ��������Xԭ�ӵĸ���=8��1/8+6��1/2=4��ÿ��������Yԭ�ӵĸ���=8�����Ըþ�������4��CaF2��������Ϊ4��78/NAg, �辧���ı߳�Ϊacm�������ܶȡ����=����, ���Ц�a3NA=4M��a3=4��78/��NA , �������������������Ca2+֮��ľ���Ϊ![]() =
=![]() ��
��![]() cm=
cm=![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�