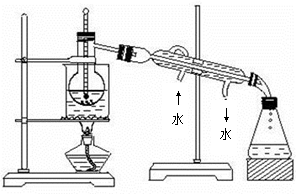
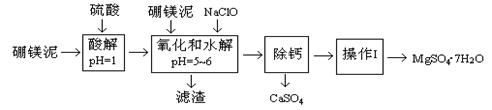
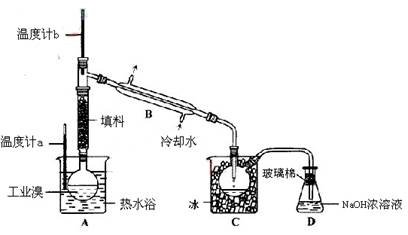
��Ŀ����
�����й�ʵ�������ȷ����
| A��ij���ʵ�ˮ��Һ��ʹ���۵⻯����Һ�����������Һһ��Ϊ��ˮ������ˮ |
| B����ij��ɫ��Һ�еμ��Ȼ�����Һ��������ɫ����������ϡ�����ó������ܽ⣬˵��ԭ��Һ��һ������SO42-���� |
| C����ij�����еμ�ϡ���ᣬ������ʹ����ʯ��ˮ����ǡ�������ʹƷ����Һ��ɫ�����壬��ԭ������һ������CO32-��HCO3-���� |
D��Ϊ�ⶨ2SO2+O2 2SO3��Ӧ���������Ԫ�ص�������������뵽���������ữ�ĸ��������Һ�У��ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ�������������㼴�� 2SO3��Ӧ���������Ԫ�ص�������������뵽���������ữ�ĸ��������Һ�У��ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ�������������㼴�� |
���������Aѡ�������Һ�п���Ϊ��ˮ��Bѡ��������ų�ԭ��Һ�к��������ӡ�Cѡ����ȷ��Dѡ��������ڼ��뵽���������ữ�ĸ��������Һ�У���Һ�к������������Ԫ���������ӡ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ