��Ŀ����
��þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO(ռ40%)������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO4��7H2O�Ĺ����������£�

��֪��NaClO��Mn2+��Ӧ����MnO2������
��������ش��������⣺
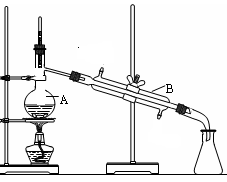
(1)ʵ��������1.00mol/L������80.0mL������98%��Ũ�������ƣ�����Ͳ������������ͷ�ι��⣬����Ҫ�IJ��������� �� ��
(2)��������Ҫ�ɷݳ�����Fe(OH)3��Al(OH)3�⣬���� �� ��
(3)�����NaClO����Mn2+��Ӧ����MnO2�������÷�Ӧ�����ӷ���ʽ�� ��
�ڵ���pH��5��6֮ǰ������һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ��
��
(4)Ϊ�˼�����Һ��Fe3+�Ƿ�������ѡ�õ��Լ��� ��
A��KSCN��Һ B������KI��Һ C��H2O2 D��KMnO4ϡ��Һ
(5)��֪MgSO4��CaSO4���ܽ�����±���
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵���������� �� ��
(6)�������ṩ����þ�������Ϊ100.0g���õ���MgSO4��7H2O196.8g����MgSO4��7H2O�IJ���Ϊ (��Է���������MgSO4��7H2O��246 MgO��40)��

��֪��NaClO��Mn2+��Ӧ����MnO2������
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 |
| ��ʼ����pH | 2.3 | 4.0 | 7.6 |
| ��ȫ����pH | 4.1 | 5.2 | 9.6 |
��������ش��������⣺
(1)ʵ��������1.00mol/L������80.0mL������98%��Ũ�������ƣ�����Ͳ������������ͷ�ι��⣬����Ҫ�IJ��������� �� ��
(2)��������Ҫ�ɷݳ�����Fe(OH)3��Al(OH)3�⣬���� �� ��
(3)�����NaClO����Mn2+��Ӧ����MnO2�������÷�Ӧ�����ӷ���ʽ�� ��
�ڵ���pH��5��6֮ǰ������һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ��
��
(4)Ϊ�˼�����Һ��Fe3+�Ƿ�������ѡ�õ��Լ��� ��
A��KSCN��Һ B������KI��Һ C��H2O2 D��KMnO4ϡ��Һ
(5)��֪MgSO4��CaSO4���ܽ�����±���
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
(6)�������ṩ����þ�������Ϊ100.0g���õ���MgSO4��7H2O196.8g����MgSO4��7H2O�IJ���Ϊ (��Է���������MgSO4��7H2O��246 MgO��40)��
��1���ձ���100mL����ƿ��2�֣� ��2��MnO2��SiO2��2�֣�
��3��Mn2+��ClO����H2O��MnO2����2H+��Cl����3�֣� 2Fe2+��ClO����2H+��2Fe3+��Cl����H2O��3�֣���4��A��2�֣� ��5������Ũ�������ȹ��ˣ�2�֣� ��6��80.0%��2�֣�д80%Ҳ���֣�
��3��Mn2+��ClO����H2O��MnO2����2H+��Cl����3�֣� 2Fe2+��ClO����2H+��2Fe3+��Cl����H2O��3�֣���4��A��2�֣� ��5������Ũ�������ȹ��ˣ�2�֣� ��6��80.0%��2�֣�д80%Ҳ���֣�
�����������1������ʵ����û��80ml����ƿ������Ҫ����1.00mol/L������80.0mL������Ҫ100ml����ƿ����������98%��Ũ�������ƣ�����Ͳ������������ͷ�ι��⣬����Ҫ�IJ����������ձ���100ml����ƿ��
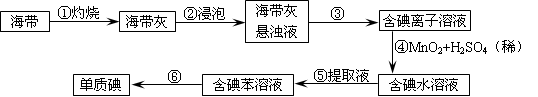
��2������NaClO��Mn2+��Ӧ����MnO2����������Ϊ�ڶ������̺Ͷ������趼����ϡ���ᷴӦ�������������д��ڣ������ֽ�л���MnO2��SiO2��
��3������NaClO��Mn2+��Ӧ����MnO2�������ڷ�Ӧ����Ԫ�صĻ��ϼ۴ӣ�1�۽��͵���1�ۣ��õ�2�����ӡ�MnԪ�صĻ��ϼ۴ӣ�2�����ߵ���4�ۣ�ʧȥ2�����ӣ�����������뻹ԭ�������ʵ���֮����1��1����NaClO��Mn2+��Ӧ����MnO2���������ӷ���ʽΪMn2+��ClO����H2O��MnO2����2H+��Cl����������Һ�����������ӣ��ܹ����������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++ClO-+2H+��2Fe3++Cl-+H2O��
��4���������ܺ�KSCN��Һ��Ӧʹ��Һ�Ժ�ɫ�����Կ�������Һ�м������軯����Һ������Һ���Ƿ�������������ӣ�����Ϊ��ȡ������Һ�������м������軯����Һ�������Һ�����ɫ��˵����Һ�в���Fe3+�������Һ���ɫ��˵����Һ�к�Fe3+��A��ȷ��������Һ�к��д������ƣ��������⻯�أ����Բ���ʹ�õ⻯�أ�B����ȷ��˫��ˮ�����Ը��������Һ������ǿ�����ԣ����ܼ��������ӣ�CD����ȷ����ѡA��
��5�������¶ȶ�����þ������Ƶ��ܽ��Ӱ�첻ͬ���¶�Խ�ߣ�������ܽ��ԽС������Ҫ��MgSO4��CaSO4�����Һ�е�CaSO4��ȥ���Բ�������Ũ�������ȹ��˷�����ȥ����ƣ���������I���ǽ���Һ��������Ũ������ȴ�ᾧ���پ������ˣ���õ���MgSO4?7H2O��
��6��100g��þ��������þ�����ʵ�����
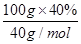 ��1mol������þԭ���غ㣬����MgSO4?7H2O�����ʵ���Ϊ1mol������Ϊ246g/mol��1mol��246g������MgSO4?7H2O�IJ��ʣ�
��1mol������þԭ���غ㣬����MgSO4?7H2O�����ʵ���Ϊ1mol������Ϊ246g/mol��1mol��246g������MgSO4?7H2O�IJ��ʣ�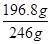 ��100%��80.0%��
��100%��80.0%��
��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ

 2SO3��Ӧ���������Ԫ�ص�������������뵽���������ữ�ĸ��������Һ�У��ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ�������������㼴��
2SO3��Ӧ���������Ԫ�ص�������������뵽���������ữ�ĸ��������Һ�У��ټ�����BaCl2��Һ�����ˡ�ϴ�ӡ�������������㼴�� ����
���� ��ɫ����
��ɫ���� ����������
���������� ��ɫ��Һ
��ɫ��Һ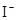 ����Һ
����Һ 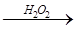 �ػ�ɫ��Һ
�ػ�ɫ��Һ 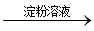 ��ɫ��Һ
��ɫ��Һ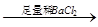 ��ɫ����
��ɫ���� �����ܽ�
�����ܽ�