��Ŀ����
8��H2O2����Ҫ�Ļ�ѧ�Լ�����ʵ���Һ�����ʵ����Ӧ�ù㷺����1��H2O2���ȶ�������������Ҫ�ϸ�������Fe2+��Fe3+������ᷢ������������Ӧ���ӿ���ֽ⣬��Fe2+��Fe3+���Ե������ֲ��䣮
��2Fe2++H2O2+2H+=2Fe3++2H2O
��2Fe3++H2O2=O2��+2Fe2++2H+�������ӷ���ʽ����
��2��H2O2��ˮ��Һ�������ԣ�д��һ����������ȡH2O2�Ļ�ѧ����ʽNa2O2+2HCl�T2NaCl+H2O2��
��3��H2O2��ʹ����ͨ�����������Ⱦ��
�ٹ�ҵ������Cl2+H2O2=2HCl+O2���ȣ��ڴ˷�Ӧ�б�������������H2O2��
������ij��Һ�е��軯���CN-����������з�Ӧ��CN-+H2O2+H2O=HCO3-+NH3��
��4��ʵ���ҳ�������KMnO4��Һ�ⶨH2O2��Ũ�ȣ�
ȡ10.00mL H2O2��Һ��Ʒ���ܶȽ���Ϊ1g/mL������ƿ�У���ˮ��50.00mL��������0.10mol/L KMnO4��Һ40.00mL��ǡ����ȫ��Ӧ����ԭ��Ʒ��H2O2������������3.4%��
���� ��1�����ݹ�������������������л�ԭ�ԣ�
��2��������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2��
��3���ٸ��ݻ��ϼ۵ı仯�жϷ�Ӧ���ͣ�
�ڸ���ԭ���غ�͵���غ������
��4�����ݷ�Ӧ��ԭ������ʽ���м��㣮
��� �⣺��1�����������������ԣ�������Fe2+��2Fe2++H2O2+2H+�T2Fe3++2H2O�����л�ԭ�ԣ��ܱ�Fe3+������2Fe3++H2O2=2Fe2++O2��+2H+���ʴ�Ϊ��2Fe3++H2O2=O2��+2Fe2++2H+��
��2��������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2������ʽΪ��Na2O2 +2HCl=2NaCl+H2O2���ʴ�Ϊ��Na2O2+2HCl�T2NaCl+H2O2��
��3����Cl2+H2O2�T2HCl+O2���÷�Ӧ��Ԫ�صĻ��ϼ۱仯Ϊ��H2O2��O2��OԪ����-1�ۡ�0�ۣ�ʧ���ӣ�����H2O2�ǻ�ԭ������ԭ������������������Ӧ��
�ʴ�Ϊ��H2O2��
����ԭ���غ�͵���غ�ã�CN-+H2O2=HCO3-+NH3�����ʴ�Ϊ��HCO3-��
��4�����ĸ������������0.1mol/L��0.04L=0.004mol����˫��ˮ�����ʵ���Ϊn����
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.004mol n
���n=0.01mol������˫��ˮ������Ϊ��0.01mol��34g/mol=0.34g��
10.00mLH2O2��Һ��Ʒ������Ϊ10g��˫��ˮ����������=$\frac{0.34g}{10g}$��100%=3.4%��
�ʴ�Ϊ��3.4%��
���� ������H2O2�����ʣ�������ѧ���������⡢���������������Ѷ��еȣ�

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�| A�� | һ����˵������Խ���ã�Խ������ʴ | |
| B�� | ����������ʴʱ���������Ե������� | |
| C�� | ����Խ�ߣ�����Խ��ʴ | |
| D�� | ������ʴͨ���ֻ�ѧ��ʴ�͵绯ѧ��ʴ���� |
| A�� | ��ǿ��ԭ�� | B�� | ֻ�������� | ||
| C�� | �ױ����� | D�� | ����涼�ܳ��ֲ�ͬ��ɫ |
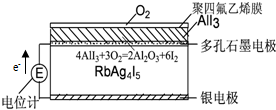
| A�� | ���Ӵ����缫��RbAg4I5������Ǩ������ʯī�缫 | |
| B�� | Ag+���������ƶ� | |
| C�� | ʯī�缫Ϊ���������缫Ϊ���� | |
| D�� | ���缫�ĵ缫��Ӧ�ǣ�Ag-e-=Ag+ |
| A�� | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ�����Ⱥ���Һ�ָ���ɫ���������һ����SO2 | |
| B�� | ��Ԫ����ɫ��Ӧ�IJ������ò�˿պȡ�Ȼ�������ú���ƵĻ����Ͻ������գ�ֱ�ӹ۲�����ɫ | |
| C�� | NH4+�ļ��飺�����Һ�м���NaOH��Һ�����ȣ�������ʹʪ�����ɫʯ����ֽ�������� | |
| D�� | ����Һ�������������ʹ����ʯ��ˮ����ǵ���ɫ���壬�����Һ��һ������CO32- |
| A�� | FeCl2 | B�� | Fe��OH��3 | C�� | NaOH | D�� | H2SiO3 |

��֪����4FeO•Cr2O3+8Na2CO3+7O2$\stackrel{750��}{��}$8Na2CrO4+2Fe2O3+8CO2��
��Na2CO3+Al2O3$\stackrel{750��}{��}$2NaAlO2+CO2��
��Cr2O72-+H2O?2CrO42-+2H+
��������ش��������⣺
��1��������������ǹ��ˣ�����X����Ҫ����Fe2O3��MgO����д��ѧʽ����
��2���ô��������ҺpH=7��8���ٽ��в��������ù���Y�ijɷ�ΪAl��OH��3��д��ѧʽ����
��3���ữ�����е�����ҺpH��5ʱ������Ӧ2CrO42-+2H+?Cr2O72-+H2O������������������ᣬ�������Cr2O72-��Ӧ��ɴ�����Ⱦ������Cr3+���ʣ��÷�Ӧ�����ӷ���ʽΪCr2O72-+6Cl-+14H+=2 Cr3++3Cl2��+7 H2O��
��4���±���������ʵ��ܽ�����ݣ�����III������Ӧ�Ļ�ѧ����ʽ��Na2Cr2O7+2KCl�TK2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ����������ǣ�K2Cr2O7���ܽ�ȱ�Na2Cr2O7С��������������K2Cr2O7���ܽ����С����
| ���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
| �ܽ�ȣ�g/100gˮ�� | 0�� | 28 | 35.7 | 4.7 | 163 |
| 40�� | 40.1 | 36.4 | 26.3 | 215 | |
| 80�� | 51.3 | 38 | 73 | 376 | |
�ٵ�ⷨ������ˮ���ܷ�Ӧ���£���ƽ����д��ȱ������֪������n��Fe��OH��3����n��H2��=1��1��
6Fe+1Cr2O72-+2H++17H2O�T6Fe��OH��3��+2Cr��OH��3��+6H2����
����֪������Cr��OH��3��Ksp=6.4��10-32mol4/l4����������������ˮˮ�ʱ������������ֵ��0.052mg/L��Ҫʹ��Һ��c��Cr3+��������������ˮˮ�ʱ����������Һ��pH����5.6������֪lg2=0.3��
| A�� | 3478Se��3480Se �ֱ���44��46������ | |
| B�� | 3478Se��3480Se��Ϊͬλ�� | |
| C�� | 3478Se ��3480Se��Ϊͬ�������� | |
| D�� | 3478Se��3480Se������34������ |

 ��
��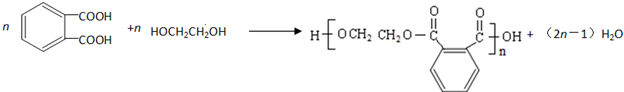 ��
��