��Ŀ����
����Ŀ������A����ͼ��ʾ��ת����ϵ(���ֲ�������ȥ)����֪H��ʹ���CCl4��Һ��ɫ��
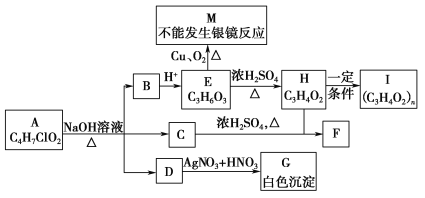
�ش��������⣺
(1)1 mol E������NaOH��Һ��Ӧʱ������NaOH�����ʵ���Ϊ________ mol��
(2)M��ijЩͬ���칹���ܷ���������Ӧ��д�����е����ֽṹ��ʽ��________________��________________��
(3)д����ѧ����ʽH�D��I��_____________________________________��C��H�D��F��____________________________________________________��
(4)Eͨ�����ɱ�ϩ��NaOH��Һ��H2��O2��Br2��Ϊԭ�Ϻϳɣ��밴��A�D��B�D��C�D����������ʽд����Ӧ���̣����ڡ��D������ע����Ӧ����__________��
���𰸡�1 OHCCH2COOH��OHCCH(OH)CHO OHCCOOCH3 nCH2=CHCOOH![]()
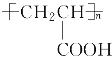 CH3OH��CH2=CHCOOH
CH3OH��CH2=CHCOOH![]() CH2===CHCOOCH3��H2O CH3CH=CH2
CH2===CHCOOCH3��H2O CH3CH=CH2![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
H��һ�������·�Ӧ����I����Ӧǰ���Ԫ�ص���ɱȲ��䣬��H��ʹ���CCl4��Һ��ɫ��˵��H��Ӧ����IӦΪ�Ӿ۷�Ӧ˵��H�к���C=C����HΪCH2=CHCOOH��IΪ![]() ���ɴ˿�֪EӦΪCH3CHOHCOOH������������
���ɴ˿�֪EӦΪCH3CHOHCOOH������������![]() �����ܷ���������Ӧ��BӦΪCH3CHOHCOONa��˵��A�ڼ�������������CH3CHOHCOONa��NaCl�ʹ����Աȷ�����ɿ�֪�ô�ΪCH3OH����AӦΪCH3CHClCOOCH3������л���Ľṹ�����ʽ����⡣
�����ܷ���������Ӧ��BӦΪCH3CHOHCOONa��˵��A�ڼ�������������CH3CHOHCOONa��NaCl�ʹ����Աȷ�����ɿ�֪�ô�ΪCH3OH����AӦΪCH3CHClCOOCH3������л���Ľṹ�����ʽ����⡣
��1��EΪCH3CHOHCOOH��������ֻ��-COOH�������ԣ�����NaOH��Ӧ����1molE��������NaOH��Һ��Ӧʱ������NaOH���ʵ���Ϊ1mol���ʴ�Ϊ��1��
��2��MΪ![]() �����Ӧ��ͬ���칹���ܷ���������Ӧ��˵�������к���CHO��HCOO-�ȹ����ţ���Ӧ��ͬ���칹�������OHCCH2COOH��OHCCH��OH��CHO��OHCCOOCH3�ȣ��ʴ�Ϊ��OHCCH2COOH��OHCCH��OH��CHO��OHCCOOCH3��
�����Ӧ��ͬ���칹���ܷ���������Ӧ��˵�������к���CHO��HCOO-�ȹ����ţ���Ӧ��ͬ���칹�������OHCCH2COOH��OHCCH��OH��CHO��OHCCOOCH3�ȣ��ʴ�Ϊ��OHCCH2COOH��OHCCH��OH��CHO��OHCCOOCH3��
��3��HΪCH2=CHCOOH���ɷ����Ӿ۷�Ӧ����![]() ����Ӧ����ʽΪ��nCH2=CHCOOH
����Ӧ����ʽΪ��nCH2=CHCOOH![]()
![]() ��CH2=CHCOOH��CH3OH����������Ӧ����CH2=CHCOOCH3����Ӧ����ʽΪCH3OH+CH2=CHCOOH
��CH2=CHCOOH��CH3OH����������Ӧ����CH2=CHCOOCH3����Ӧ����ʽΪCH3OH+CH2=CHCOOH![]() CH2=CHCOOCH3+H2O���ʴ�Ϊ��nCH2=CHCOOH
CH2=CHCOOCH3+H2O���ʴ�Ϊ��nCH2=CHCOOH![]()
![]() ��CH3OH+CH2=CHCOOH
��CH3OH+CH2=CHCOOH![]() CH2=CHCOOCH3+H2O��
CH2=CHCOOCH3+H2O��
��4���ɱ�ϩ��NaOH��Һ��H2��O2��Br2��Ϊԭ�Ϻϳ�CH3CHOHCOOH�����漰���·�Ӧ���̣�CH3CH=CH2![]() CH3CHBrCH2Br
CH3CHBrCH2Br![]() CH3CHOHCH2OH
CH3CHOHCH2OH![]()
![]()
![]()
![]()
![]()
![]() ���ʴ�Ϊ��CH3CH=CH2
���ʴ�Ϊ��CH3CH=CH2![]() CH3CHBrCH2Br
CH3CHBrCH2Br![]() CH3CHOHCH2OH
CH3CHOHCH2OH![]()
![]()
![]()
![]()
![]()
![]() ��
��

����Ŀ��I. ����ʵ�鷽����������________��
A������������Һ����һ����AgNO3��Һ�У��μӰ�ˮ������ǡ���ܽ�
B���ڼ���ȩ������Cu(OH)2����Һʱ����һ����CuSO4��Һ�У���������NaOH��Һ
C����֤RXΪ����飬��RX���ռ�ˮ��Һ��ϼ��ȣ�����Һ��ȴ���ټ�����������Һ
D����ˮ�Ҵ���Ũ���Ṳ�ȵ�170 �棬���Ƶõ�����ͨ�����Ը�����أ��ɼ����Ƶõ������Ƿ�Ϊ��ϩ
E�������л�����ϩ��ͨ��������һ�������·�Ӧ��ʹ��ϩת��Ϊ����
F����ȥ���ڱ��е��������ӣ����������ˮ������
G��ʵ������ȡ��ϩʱ���뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ���¶�
II.ij��ѧС���������������������װ��(����ͼ)���Ի������Ʊ�����ϩ��
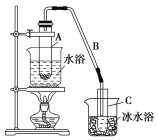
��֪��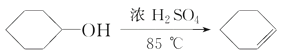 +H2O
+H2O
�ܶ�(g��cm��3) | �۵�(��) | �е�(��) | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | ��103 | 83 | ������ˮ |
(1)�Ʊ���Ʒ��
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������________________���ڵ���B���˵�������е�������__________��
(2)�Ʊ���Ʒ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________(��ϡ����¡�)�㣬��Һ����______(����)ϴ�ӡ�
a��KMnO4��Һ�� b��ϡH2SO4�� c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��________(�f����g��)�ڽ��롣
����Ŀ����1L��0.01molNaAlO2��0.02molNaOH����Һ�л���ͨ�������̼����n(CO2)�����Ⱥ���������ͬ�ķ�Ӧ����0.01mol<n(CO2) ![]() 0.015ʱ�����ķ�Ӧ�ǣ�2 NaAlO2+ CO2+2H2O=2Al(OH)3��+Na2CO3���ж�Ӧ��ϵ��ȷ����( )
0.015ʱ�����ķ�Ӧ�ǣ�2 NaAlO2+ CO2+2H2O=2Al(OH)3��+Na2CO3���ж�Ӧ��ϵ��ȷ����( )
ѡ�� | n(CO2)/mol | ��Һ�����ӵ����ʵ���Ũ�� |
A | 0 | c(Na+)>c(AlO2-)+c(OH-) |
B | 0.01 | c(Na+)>c(AlO2-)> c(OH-)>c(CO32-) |
C | 0.015 | c(Na+)> c(HCO3-)>c(CO32-)> c(OH-) |
D | 0.03 | c(Na+)> c(HCO3-)> c(OH-)>c(H+) |
A. A B. B C. C D. D
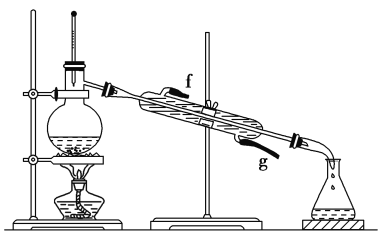
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿװ�ã������װ�ûش����⣺
(1)��װ��A��װ��B�ж��õ���������װ��A�в�������������___��
(2)װ��C�Тٵ�������___����װ��������ˮ����������___(�����Ͻ��³����������½��ϳ���)��װ��D�еķ�Һ©����ʹ��֮ǰӦ��___���ڷ�ҺʱΪʹҺ��˳�����£�Ӧ���еľ��������___��
(3)ij�����ƹ����л����������������ʣ������һʵ�鷽�����ȳ�ȥ���ʣ��������������Һ��ʵ�鷽�����Ƚ�������������ˮ�����Һ��ѡ����ʵ��Լ��Ͳ�����ɱ����и���ʵ�顣
ѡ���ʵ��Լ���˳������ | �� | Na2CO3��Һ | �� |
ʵ����� | �� | �� | ���� |
��������Լ��ٿ�����___(�ѧʽ)��֤����Һ��SO42-�Ѿ������ķ�����___������Na2CO3��Һ��Ŀ����___����������Լ��ܿ�����___(�ѧʽ)��