��Ŀ����
A��B��C��D��E��F�Ƕ�����Ԫ�أ����ڱ���A��B��B��C���ڣ�C��Eͬ���壻A��C����������֮��Ϊ2:3��B��������������C��������������1���� FԪ�ص�ԭ�������ڱ��а뾶��С������������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һʹ��̪��Һ��졣
��1��E�����ڱ��е�λ��Ϊ__________________________��D������������ˮ����ĵ���ʽ___________________________��
��2��B��C���⻯���ȶ���˳��Ϊ���÷���ʽ��ʾ���ɴ�С��_________��__________��
��3��B���⻯���B������������ˮ���ﷴӦ����Z����Z������Ϊ____________________��Z�Ļ�ѧ������Ϊ____________________��
��4�����־���C��D��E��F����Ԫ�صĻ��������Ӧ�ų�����ķ�Ӧ���ӷ���ʽΪ_____________________________________��
��5��һ������D2C2��AC2��Ӧ��Ĺ������ʣ�ǡ���뺬0.8mol HCl��ϡ������ȫ��Ӧ�����ռ�0.35 mol ���壬��������ʵ����Ϊ��д��ɷֺ����ʵ�����____________________��
��1��E�����ڱ��е�λ��Ϊ__________________________��D������������ˮ����ĵ���ʽ___________________________��
��2��B��C���⻯���ȶ���˳��Ϊ���÷���ʽ��ʾ���ɴ�С��_________��__________��
��3��B���⻯���B������������ˮ���ﷴӦ����Z����Z������Ϊ____________________��Z�Ļ�ѧ������Ϊ____________________��
��4�����־���C��D��E��F����Ԫ�صĻ��������Ӧ�ų�����ķ�Ӧ���ӷ���ʽΪ_____________________________________��
��5��һ������D2C2��AC2��Ӧ��Ĺ������ʣ�ǡ���뺬0.8mol HCl��ϡ������ȫ��Ӧ�����ռ�0.35 mol ���壬��������ʵ����Ϊ��д��ɷֺ����ʵ�����____________________��
��15�֣������һ��3�֣�����ÿ��2�֣�
��1���������ڵڢ�A��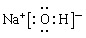
��2��H2O ��NH3
��3������� ���Ӽ������ۼ�
��4��HSO3�� +H+ = H2O+SO2 ��
��5��0.1mol Na2O2 0.3mol Na2CO3
��1���������ڵڢ�A��
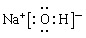
��2��H2O ��NH3
��3������� ���Ӽ������ۼ�
��4��HSO3�� +H+ = H2O+SO2 ��
��5��0.1mol Na2O2 0.3mol Na2CO3
�������������������Ϣ������FԪ�ص�ԭ�������ڱ��а뾶��С��FΪHԪ�ء�����������D2C2��ˮ��Ӧ����C�ĵ��ʣ�����Һʹ��̪��Һ��죬D2C2ΪNa2O2��CΪOԪ�أ�DΪNaԪ�ء�B��C���ڣ�B��������������C��������������1����BΪNԪ�ء�C��Eͬ���壬EΪSԪ�ء�A��B���ڣ�A��C����������֮��Ϊ2:3��AΪCԪ�ء�
��1��EΪSԪ�أ������ڱ��е�λ��Ϊ�������ڵڢ�A�塣DΪNaԪ�أ�����������ˮ����ΪNaOH������ʽΪ
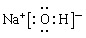 ��
����2��BΪNԪ�أ�CΪOԪ�أ��⻯��ֱ�ΪNH3��H2O��OԪ�صķǽ����Դ���NԪ�أ��⻯���ȶ���ΪH2O ��NH3��
��3��BΪNԪ�أ��⻯��ֱ�ΪNH3������������ˮ������HNO3��NH3��HNO3��Ӧ����NH4NO3��Z������Ϊ����泥��������Ӽ����ۼ���
��4��C��D��E��F�ֱ�ΪOԪ�ء�NaԪ�ء�SԪ�ء�HԪ�ء�����Ԫ�صĻ��������Ӧ�ų����������ΪNaHSO3��H2SO4����Ӧ�����ӷ���ʽΪHSO3�� +H+ = H2O+SO2��
��5��D2C2��AC2�ֱ�ΪNa2O2��CO2����Ӧ����Na2CO3��O2������Na2CO3��2H+��CO2���������2Na2O2��4H+��O2��������������ΪNa2O2��Na2CO3�Ļ�Ϲ��壬��2n(Na2CO3)+2n(Na2O2)=0.8mol��n(Na2CO3)+1/2n(Na2O2)=0.35mol�����n(Na2CO3)= 0.3mol��n(Na2O2)="0.1" mol��
���������⿼��Ԫ�ص��ƶ��Լ�Ԫ�ػ�����֪ʶ�������ʱע�����ԭ�ӵĽṹ�ص㣬���Ԫ��������֪ʶ�����ƶϣ������Ѷ��еȡ�

��ϰ��ϵ�д�
�����Ŀ
 ��YΪ
��YΪ
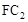 �����ж����ŷŵ����������γ����꣬д��
�����ж����ŷŵ����������γ����꣬д��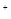 )��c(OH
)��c(OH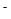 )
)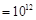 ����Һ�У�
����Һ�У�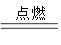 A+N2��+3CO2��������ƽ��
A+N2��+3CO2��������ƽ��